Abstract
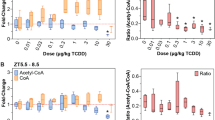
Cinnamic acid and its analogues (pyragrel and ozagrel) undergo chain-shortened (β-oxidative) and reductive metabolism on acyl side chain. In this study, we characterized the β-oxidative and reductive metabolism on acyl side chain of cinnamic acid and its analogues using primary rat hepatocytes, hepatic mitochondrial, and microsomal systems. A compartmental model including parent compounds and metabolites was developed to characterize in vivo β-oxidative and reductive metabolism following an intravenous dose of parent compounds to rats. The fitted total in vivo clearance values were further compared with the in vitro values predicted by the well-stirred model. We showed that hepatic microsomal CYP450s did not catalyze β-oxidative or reductive metabolism of the three compounds. Similar to β-oxidation of fatty acids, β-oxidative metabolism on their acyl side chain occurred mainly in mitochondria, which was highly dependent on ATP, CoA and NAD+. Fatty acids and NADH inhibited the β-oxidative metabolism. Reductive metabolism occurred in both mitochondria and microsomes. Reduction in mitochondria was ATP-, CoA-, and NAD(P)H-dependent and reversible, which was suppressed by enoyl reductase inhibitor triclosan. Reduction in microsomes was ATP-, CoA-, and NADPH-dependent but little affected by triclosan. Both plasma concentrations of β-oxidative metabolites and reductive metabolites were successfully fitted using the compartmental model. The estimated total in vivo clearance values were consistent with those predicted from hepatocytes and organelles, implicating significance of in vitro kinetics. These findings demonstrate the roles of hepatic mitochondria and microsomes in β-oxidative and reductive metabolism on acyl side chain of cinnamic acid and its analogues along with their metabolic characteristics.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Fang W, Wei J, Han D, Chen X, He G, Wu Q, et al. MC-002 exhibits positive effects against platelets aggregation and endothelial dysfunction through thromboxane A2 inhibition. Thromb Res. 2014;133:610–5.
Zhao X, Jiang J, Yang G, Huang J, Yang G, He G, et al. Profiling and preparation of metabolites from pyragrel in human urine by online solid-phase extraction coupled with high performance liquid chromatography tandem mass spectrometry followed by a macroporous resin-based purification approach. Molecules. 2017;22:E494.
Yang G, Pei Q, Fu C, Huang J, Chen J, Yang S, et al. Simultaneous determination of Pyragrel, a novel anti-thrombotic agent, and its two primary metabolites in plasma by HPLC-MS/MS. J Pharm Biomed Anal. 2018;156:199–205.
Nutley BP, Farmer P, Caldwell J. Metabolism of trans-cinnamic acid in the rat and the mouse and its variation with dose. Food Chem Toxicol. 1994;32:877–86.
Oguro H, Mitaki S, Takayoshi H, Abe S, Onoda K, Yamaguchi S. Retrospective analysis of argatroban in 353 patients with acute noncardioembolic stroke. J Stroke Cerebrovasc Dis. 2018;27:2175–81.
Ogiso T, Iwaki M, Hara Y, Tanino T. Pharmacokinetics of ozagrel and its metabolites after intravenous and oral administrations. J Pharm Sci. 1997;86:1111–4.
Hoskins JA. The occurrence, metabolism and toxicity of cinnamic acid and related compounds. J Appl Toxicol. 1984;4:283–92.
Seubert W, Podack ER. Mechanisms and physiological roles of fatty acid chain elongation in microsomes and mitochondria. Mol Cell Biochem. 1973;1:29–40.
Ranganathan S, Ramasarma T. The metabolism of phenolic acids in the rat. Biochem J. 1974;140:517–22.
Li F, Zhang M, Xu D, Liu C, Zhong ZY, Jia LL, et al. Co-administration of paroxetine and pravastatin causes deregulation of glucose homeostasis in diabetic rats via enhanced paroxetine exposure. Acta Pharmacol Sin. 2014;35:792–805.
Kemp DC, Brouwer KL. Viability assessment in sandwich-cultured rat hepatocytes after xenobiotic exposure. Toxicol Vitr. 2004;18:869–77.
Bjorge SM, Baillie TA. Studies on the beta-oxidation of valproic acid in rat liver mitochondrial preparations. Drug Metab Dispos. 1991;19:823–9.
Hu N, Xie S, Liu L, Wang X, Pan X, Chen G, et al. Opposite effect of diabetes mellitus induced by streptozotocin on oral and intravenous pharmacokinetics of verapamil in rats. Drug Metab Dispos. 2011;39:419–25.
Lenartowicz E, Winter C, Kunz W, Wojtczak AB. The inhibition of isocitrate oxidation by palmitoyl-l-carnitine and palmitoyl-CoA in rat liver mitochondria. Eur J Biochem. 1976;67:137–44.
Yang X, Lu J, Ying M, Mu J, Li P, Liu Y. Docking and molecular dynamics studies on triclosan derivatives binding to FabI. J Mol Model. 2017;23:25.
Sippel KH, Vyas NK, Zhang W, Sankaran B, Quiocho FA. Crystal structure of the human fatty acid synthase enoyl-acyl carrier protein-reductase domain complexed with triclosan reveals allosteric protein-protein interface inhibition. J Biol Chem. 2014;289:33287–95.
Liu B, Wang Y, Fillgrove KL, Anderson VE. Triclosan inhibits enoyl-reductase of type I fatty acid synthase in vitro and is cytotoxic to MCF-7 and SKBr-3 breast cancer cells. Cancer Chemother Pharmacol. 2002;49:187–93.
Wu J, Jin Z, Zheng H, Yan LJ. Sources and implications of NADH/NAD(+) redox imbalance in diabetes and its complications. Diabetes Metab Syndr Obes. 2016;9:145–53.
Diaz-Flores M, Ibanez-Hernandez MA, Galvan RE, Gutierrez M, Duran-Reyes G, Medina-Navarro R, et al. Glucose-6-phosphate dehydrogenase activity and NADPH/NADP+ratio in liver and pancreas are dependent on the severity of hyperglycemia in rat. Life Sci. 2006;78:2601–7.
Dou YH, Wang L, He GW, Wu Q, Fan GR, Hang TJ. Determination of (E)-3-(3-methoxy-4-((3,5,6-trimethylpyrazin-2-yl)methoxy)phenyl)acrylate in rat plasma by RP-HPLC. J Pharm Pract. 2012;30:42–4, 48.
Yang BK, Wang SJ, Mo LL, Zeng J, Zhong YM, Zang LQ. Studies on the absolute bioavailability of cinnamic acid in rats and its absorption properties. PharmJChinPLA. 2013;29:13–6.
Xu D, Li F, Zhang M, Zhang J, Liu C, Hu MY, et al. Decreased exposure of simvastatin and simvastatin acid in a rat model of type 2 diabetes. Acta Pharmacol Sin. 2014;35:1215–25.
Naritomi Y, Terashita S, Kimura S, Suzuki A, Kagayama A, Sugiyama Y. Prediction of human hepatic clearance from in vivo animal experiments and in vitro metabolic studies with liver microsomes from animals and humans. Drug Metab Dispos. 2001;29:1316–24.
Guest EJ, Aarons L, Houston JB, Rostami-Hodjegan A, Galetin A. Critique of the two-fold measure of prediction success for ratios: application for the assessment of drug-drug interactions. Drug Metab Dispos. 2011;39:170–3.
Parrott N, Paquereau N, Coassolo P, Lave T. An evaluation of the utility of physiologically based models of pharmacokinetics in early drug discovery. J Pharm Sci. 2005;94:2327–43.
Bustamante E, Soper JW, Pedersen PL. A high-yield preparative method for isolation of rat liver mitochondria. Anal Biochem. 1977;80:401–8.
Poet TS, Wu H, English JC, Corley RA. Metabolic rate constants for hydroquinone in F344 rat and human liver isolated hepatocytes: application to a PBPK model. Toxicol Sci. 2004;82:9–25.
Chen Y, Ma Y, Ma W. Pharmacokinetics and bioavailability of cinnamic acid after oral administration of Ramulus Cinnamomi in rats. Eur J Drug Metab Pharmacokinet. 2009;34:51–6.
Guengerich FP. Cytochrome p450 and chemical toxicology. Chem Res Toxicol. 2008;21:70–83.
Fukami MH, Williamson JR. On the mechanism of inhibition of fatty acid oxidation by 4-pentenoic acid in rat liver mitochondria. J Biol Chem. 1971;246:1206–12.
Suzue G, Marcel YL. Kinetic studies on the chain length specificity of long chain acyl coenzyme A synthetase from rat liver microsomes. J Biol Chem. 1972;247:6781–3.
Krisans SK, Mortensen RM, Lazarow PB. Acyl-CoA synthetase in rat liver peroxisomes. Computer-assisted analysis of cell fractionation experiments. J Biol Chem. 1980;255:9599–607.
Lageweg W, Wanders RJ, Tager JM. Long-chain-acyl-CoA synthetase and very-long-chain-acyl-CoA synthetase activities in peroxisomes and microsomes from rat liver. An enzymological study. Eur J Biochem. 1991;196:519–23.
Lewin TM, Van Horn CG, Krisans SK, Coleman RA. Rat liver acyl-CoA synthetase 4 is a peripheral-membrane protein located in two distinct subcellular organelles, peroxisomes, and mitochondrial-associated membrane. Arch Biochem Biophys. 2002;404:263–70.
Mannaerts GP, Debeer LJ. Mitochondrial and peroxisomal beta-oxidation of fatty acids in rat liver. Ann N Y Acad Sci. 1982;386:30–9.
Yamada J, Horie S, Watanabe T, Suga T. Participation of peroxisomal beta-oxidation system in the chain-shortening of a xenobiotic acyl compound. Biochem Biophys Res Commun. 1984;125:123–8.
Schonfeld P, Wojtczak L. Short- and medium-chain fatty acids in energy metabolism: the cellular perspective. J Lipid Res. 2016;57:943–54.
Bremer J. Carnitine–metabolism and functions. Physiol Rev. 1983;63:1420–80.
Smeland TE, Nada M, Cuebas D, Schulz H. NADPH-dependent beta-oxidation of unsaturated fatty acids with double bonds extending from odd-numbered carbon atoms. Proc Natl Acad Sci USA. 1992;89:6673–7.
Nagi MN, Prasad MR, Cook L, Cinti DL. Biochemical properties of short- and long-chain rat liver microsomal trans-2-enoyl coenzyme A reductase. Arch Biochem Biophys. 1983;226:50–64.
Prasad MR, Chiang CF, Cook L, Cinti DL. Solubilization and purification of hepatic microsomal trans-2-enoyl-CoA reductase: evidence for the existence of a second long-chain enoyl-CoA reductase. Arch Biochem Biophys. 1985;237:535–44.
Hinsch W, Klages C, Seubert W. On the mechanism of malonyl-CoA-independent fatty-acid synthesis. Different properties of the mitochondrial chain elongation and enoylCoA reductase in various tissues. Eur J Biochem. 1976;64:45–55.
Prasad MR, Nagi MN, Cook L, Cinti DL. Kinetic evidence for two separate trans-2-enoyl CoA reductases in rat hepatic microsomes: NADPH-specific short chain- and NAD(P)H-dependent long chain-reductase. Biochem Biophys Res Commun. 1983;113:659–65.
Cinti DL, Nagi MN, Cook L, White RE. Evidence for a second microsomal trans-2-enoyl coenzyme A reductase in rat liver. NADPH-Specif Short chain reductase J Biol Chem. 1982;257:14333–40.
Farinelli E, Giampaoli D, Cenciarini A, Cercado E, Verrotti A. Valproic acid and nonalcoholic fatty liver disease: A possible association? World J Hepatol. 2015;7:1251–7.
Fromenty B, Freneaux E, Labbe G, Deschamps D, Larrey D, Letteron P, et al. Tianeptine, a new tricyclic antidepressant metabolized by beta-oxidation of its heptanoic side chain, inhibits the mitochondrial oxidation of medium and short chain fatty acids in mice. Biochem Pharmacol. 1989;38:3743–51.
Panel RE, Belsito D, Bickers D, Bruze M, Calow P, Dagli M, et al. A toxicologic and dermatologic assessment of cinnamyl phenylpropyl materials when used as fragrance ingredients. Food Chem Toxicol. 2011;49(Suppl 2):S256–67.
Sasaki S, Kitamura S, Negoro N, Suzuki M, Tsujihata Y, Suzuki N, et al. Design, synthesis, and biological activity of potent and orally available G protein-coupled receptor 40 agonists. J Med Chem. 2011;54:1365–78.
Negoro N, Sasaki S, Ito M, Kitamura S, Tsujihata Y, Ito R, et al. Identification of fused-ring alkanoic acids with improved pharmacokinetic profiles that act as G protein-coupled receptor 40/free fatty acid receptor 1 agonists. J Med Chem. 2012;55:1538–52.
Acknowledgements
The project was supported by the National Natural Science Foundation of China (No. 81573490, 81872930, 81473273 and 81673505); Natural Science Foundation of Jiangsu Province of China (BK20161457); and the “333”, “Six Talent Peaks” and “Cyan Blue” Projects of Jiangsu Province. We thank Hefei Industrial Pharmaceutical Institute Co., Ltd. (Hefei, China) for kindly providing the pyragrel and its two metabolites and the Department of Medicinal Chemistry of China Pharmaceutical University (Nanjing, China) kindly providing the ozagrel-M2.
Author contributions
K-jZ and X-dL designed the experiments, analyzed the data, and wrote the manuscript; K-jZ, YC, S-jH, Y-tY, JX, H-yY, LZ, X-gT, Q-sX, T-tY and Y-qZ conducted the experiments; K-jZ and YC performed the data analysis and ML and LL reviewed the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary Information
Rights and permissions
About this article
Cite this article
Zhao, Kj., Chen, Y., Hong, Sj. et al. Characteristics of β-oxidative and reductive metabolism on the acyl side chain of cinnamic acid and its analogues in rats. Acta Pharmacol Sin 40, 1106–1118 (2019). https://doi.org/10.1038/s41401-019-0218-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-019-0218-8
Keywords
This article is cited by
-
Indoxyl sulphate-TNFα axis mediates uremic encephalopathy in rodent acute kidney injury
Acta Pharmacologica Sinica (2024)