Abstract
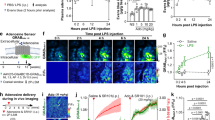
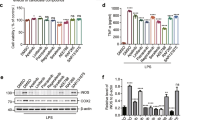
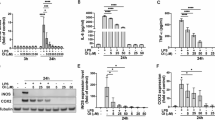
Neuroinflammation is one of the critical events in neurodegenerative diseases, whereas microglia play an important role in the pathogenesis of neuroinflammation. In this study, we investigated the effects of a natural sesquiterpene lactone, 6-O-angeloylplenolin (6-OAP), isolated from the traditional Chinese medicine Centipeda minima (L.) A.Br., on neuroinflammation and the underlying mechanisms. We showed that treatment with lipopolysaccharide (LPS) caused activation of BV2 and primary microglial cells and development of neuroinflammation in vitro, evidenced by increased production of inflammatory cytokines TNF-α and IL-1β, the phosphorylation and nuclear translocation of NF-κB, and the transcriptional upregulation of COX-2 and iNOS, leading to increased production of proinflammatory factors NO and PGE2. Moreover, LPS treatment induced oxidative stress through increasing the expression levels of NOX2 and NOX4. Pretreatment with 6-OAP (0.5−4 μM) dose-dependently attenuated LPS-induced NF-κB activation and oxidative stress, thus suppressed neuroinflammation in the cells. In a mouse model of LPS-induced neuroinflammation, 6-OAP (5−20 mg·kg−1·d−1, ip, for 7 days before LPS injection) dose-dependently inhibited the production of inflammatory cytokines, the activation of the NF-κB signaling pathway, and the expression of inflammatory enzymes in brain tissues. 6-OAP pretreatment significantly ameliorated the activation of microglia and astrocytes in the brains. 6-OAP at a high dose caused a much stronger antineuroinflammatory effect than dexamethansone (DEX). Furthermore, we demonstrated that 6-OAP pretreatment could inhibit LPS-induced neurite and synaptic loss in vitro and in vivo. In conclusion, our results demonstrate that 6-OAP exerts antineuroinflammatory effects and can be considered a novel drug candidate for the treatment of neuroinflammatory diseases.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Amor S, Puentes F, Baker D, van der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129:154–69.
Eikelenboom P, Bate C, Van Gool WA, Hoozemans JJ, Rozemuller JM, Veerhuis R, Williams A. Neuroinflammation in Alzheimer’s disease and prion disease. Glia. 2002;40:232–39.
Kim YS, Joh TH. Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson’s disease. Exp Mol Med. 2006;38:333–47.
McGeer EG, McGeer PL. Inflammatory processes in Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:741–49.
Sanders P, De Keyser J. Janus faces of microglia in multiple sclerosis. Brain Res Rev. 2007;54:274–85.
Liu B, Hong JS. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther. 2003;304:1–7.
Al-Amin MM, Reza HM. Neuroinflammation: contemporary anti-inflammatory treatment approaches. Neurosci (Riyadh). 2014;19:87–92.
Walsh JG, Muruve DA, Power C. Inflammasomes in the CNS. Nat Rev Neurosci. 2014;15:84–97.
Whitney NP, Eidem TM, Peng H, Huang Y, Zheng JC. Inflammation mediates varying effects in neurogenesis: relevance to the pathogenesis of brain injury and neurodegenerative disorders. J Neurochem. 2009;108:1343–59.
Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69.
Morales I, Guzman-Martinez L, Cerda-Troncoso C, Farias GA, Maccioni RB. Neuroinflammation in the pathogenesis of Alzheimer’s disease. A rational framework for the search of novel therapeutic approaches. Front Cell Neurosci. 2014;8:112.
Siopi E, Llufriu-Daben G, Cho AH, Vidal-Lletjos S, Plotkine M, Marchand-Leroux C, et al. Etazolate, an alpha-secretase activator, reduces neuroinflammation and offers persistent neuroprotection following traumatic brain injury in mice. Neuropharmacology. 2013;67:183–92.
Brown J, Wang H, Hajishengallis GN, Martin M. TLR-signaling networks: an integration of adaptor molecules, kinases, and cross-talk. J Dent Res. 2011;90:417–27.
Yao L, Kan EM, Lu J, Hao A, Dheen ST, Kaur C, Ling EA. Toll-like receptor 4 mediates microglial activation and production of inflammatory mediators in neonatal rat brain following hypoxia: role of TLR4 in hypoxic microglia. J Neuroinflamm. 2013;10:23.
Berger T, Saunders ME, Mak TW. Dissection of signaling in inflammation: three novel inflammatory regulators. Cold Spring Harb Symp Quant Biol. 2013;78:141–47.
Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–62.
Saha RN, Pahan K. Regulation of inducible nitric oxide synthase gene in glial cells. Antioxid Redox Signal. 2006;8:929–47.
von Bernhardi R, Eugenin-von Bernhardi L, Eugenin J. Microglial cell dysregulation in brain aging and neurodegeneration. Front Aging Neurosci. 2015;7:124.
Woodling NS, Andreasson KI. Untangling the web: toxic and protective effects of neuroinflammation and PGE2 signaling in Alzheimer’s disease. ACS Chem Neurosci. 2016;7:454–63.
Ben Mkaddem S, Pedruzzi E, Werts C, Coant N, Bens M, Cluzeaud F, et al. Heat shock protein gp96 and NAD(P)H oxidase 4 play key roles in Toll-like receptor 4-activated apoptosis during renal ischemia/reperfusion injury. Cell Death Differ. 2010;17:1474–85.
Brown GC. Mechanisms of inflammatory neurodegeneration: iNOS and NADPH oxidase. Biochem Soc Trans. 2007;35:1119–21.
Li Q, Spencer NY, Oakley FD, Buettner GR, Engelhardt JF. Endosomal Nox2 facilitates redox-dependent induction of NF-kappaB by TNF-alpha. Antioxid Redox Signal. 2009;11:1249–63.
Rojo AI, McBean G, Cindric M, Egea J, Lopez MG, Rada P, et al. Redox control of microglial function: molecular mechanisms and functional significance. Antioxid Redox Signal. 2014;21:1766–801.
Anrather J, Racchumi G, Iadecola C. NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J Biol Chem. 2006;281:5657–67.
Gauss KA, Nelson-Overton LK, Siemsen DW, Gao Y, DeLeo FR, Quinn MT. Role of NF-kappaB in transcriptional regulation of the phagocyte NADPH oxidase by tumor necrosis factor-alpha. J Leukoc Biol. 2007;82:729–41.
Nayernia Z, Jaquet V, Krause KH. New insights on NOX enzymes in the central nervous system. Antioxid Redox Signal. 2014;20:2815–37.
Choi DK, Koppula S, Suk K. Inhibitors of microglial neurotoxicity: focus on natural products. Molecules. 2011;16:1021–43.
Shal B, Ding W, Ali H, Kim YS, Khan S. Anti-neuroinflammatory potential of natural products in attenuation of Alzheimer’s disease. Front Pharmacol. 2018;9:548.
Suk K, Ock J. Chemical genetics of neuroinflammation: natural and synthetic compounds as microglial inhibitors. Inflammopharmacology. 2012;20:151–8.
Schwikkard S, van Heerden FR. Antimalarial activity of plant metabolites. Nat Prod Rep. 2002;19:675–92.
Taylor RS, Towers GH. Antibacterial constituents of the Nepalese medicinal herb, Centipeda minima. Phytochemistry. 1998;47:631–4.
Huang SS, Chiu CS, Lin TH, Lee MM, Lee CY, Chang SJ, et al. Antioxidant and anti-inflammatory activities of aqueous extract of Centipeda minima. J Ethnopharmacol. 2013;147:395–405.
Liu ZG, Yu HM, Wen SL, Liu YL. Histopathological study on allergic rhinitis treated with Centipeda minima. Zhongguo Zhong Yao Za Zhi. 2005;30:292–4.
Wu JB, Chun YT, Ebizuka Y, Sankawa U. Biologically active constituents of Centipeda minima: sesquiterpenes of potential anti-allergy activity. Chem Pharm Bull (Tokyo). 1991;39:3272–5.
Liang H, Bao F, Dong X, Tan R, Zhang C, Lu Q, et al. Antibacterial thymol derivatives isolated from Centipeda minima. Molecules. 2007;12:1606–13.
Liang HX, Bao FK, Dong XP, Zhu HJ, Lu XJ, Shi M, et al. Two new antibacterial sesquiterpenoids from Centipeda minima. Chem Biodivers. 2007;4:2810–6.
Wu P, Li XG, Liang N, Wang GC, Ye WC, Zhou GX, et al. Two new sesquiterpene lactones from the supercritical fluid extract of Centipeda minima. J Asian Nat Prod Res. 2012;14:515–20.
Wu P, Su MX, Wang Y, Wang GC, Ye WC, Chung HY, et al. Supercritical fluid extraction assisted isolation of sesquiterpene lactones with antiproliferative effects from Centipeda minima. Phytochemistry. 2012;76:133–40.
Ding LF, Liu Y, Liang HX, Liu DP, Zhou GB, Cheng YX. Two new terpene glucosides and antitumor agents from Centipeda minima. J Asian Nat Prod Res. 2009;11:732–36.
Oh HM, Kwon BM, Baek NI, Kim SH, Lee JH, Eun JS, et al. Inhibitory activity of 6-O-angeloylprenolin from Centipeda minima on farnesyl protein transferase. Arch Pharm Res. 2006;29:64–6.
Liu Y, Chen XQ, Liang HX, Zhang FX, Zhang B, Jin J, et al. Small compound 6-O-angeloylplenolin induces mitotic arrest and exhibits therapeutic potentials in multiple myeloma. PLoS ONE. 2011;6:e21930.
Liu YQ, Wang XL, Cheng X, Lu YZ, Wang GZ, Li XC, et al. Skp1 in lung cancer: clinical significance and therapeutic efficacy of its small molecule inhibitors. Oncotarget. 2015;6:34953–67.
Saura J, Tusell JM, Serratosa J. High-yield isolation of murine microglia by mild trypsinization. Glia. 2003;44:183–89.
Li Y, Wang J, Sheng JG, Liu L, Barger SW, Jones RA, et al. S100 beta increases levels of beta-amyloid precursor protein and its encoding mRNA in rat neuronal cultures. J Neurochem. 1998;71:1421–8.
Meijering E, Jacob M, Sarria JC, Steiner P, Hirling H, Unser M. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytom A. 2004;58:167–76.
Tam WY, Ma CH. Bipolar/rod-shaped microglia are proliferating microglia with distinct M1/M2 phenotypes. Sci Rep. 2014;4:7279.
Chuang KA, Li MH, Lin NH, Chang CH, Lu IH, Pan IH, et al. Rhinacanthin C alleviates amyloid-beta fibrils’ toxicity on neurons and attenuates neuroinflammation triggered by LPS, amyloid-beta, and interferon-gamma in glial cells. Oxid Med Cell Longev. 2017;2017:5414297.
Wu JB, Chun YT, Ebizuka Y, Sankawa U. Biologically active constituents of Centipeda minima: isolation of a new plenolin ester and the antiallergy activity of sesquiterpene lactones. Chem Pharm Bull (Tokyo). 1985;33:4091–4.
Su M, Li Y, Chung HY, Ye W. 2beta-(Isobutyryloxy)florilenalin, a sesquiterpene lactone isolated from the medicinal plant Centipeda minima, induces apoptosis in human nasopharyngeal carcinoma CNE cells. Molecules. 2009;14:2135–46.
Huang W, Yu X, Liang N, Ge W, Kwok HF, Lau CB, et al. Anti-angiogenic activity and mechanism of sesquiterpene lactones from Centipeda minima. Nat Prod Commun. 2016;11:435–8.
Zandi PP, Anthony JC, Hayden KM, Mehta K, Mayer L, Breitner JC, et al. Reduced incidence of AD with NSAID but not H2 receptor antagonists: the Cache County Study. Neurology. 2002;59:880–6.
Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, et al. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA. 2003;289:2819–26.
Kroot EJ, Huisman AM, Van Zeben J, Wouters JM, Van Paassen HC. Oral pulsed dexamethasone therapy in early rheumatoid arthritis: a pilot study. Ann N Y Acad Sci. 2006;1069:300–6.
Meneses G, Gevorkian G, Florentino A, Bautista MA, Espinosa A, Acero G, et al. Intranasal delivery of dexamethasone efficiently controls LPS-induced murine neuroinflammation. Clin Exp Immunol. 2017;190:304–14.
Wang J, Song Y, Chen Z, Leng SX. Connection between systemic inflammation and neuroinflammation underlies neuroprotective mechanism of several phytochemicals in neurodegenerative diseases. Oxid Med Cell Longev. 2018;2018:1972714.
Fu HQ, Yang T, Xiao W, Fan L, Wu Y, Terrando N, et al. Prolonged neuroinflammation after lipopolysaccharide exposure in aged rats. PLoS ONE. 2014;9:e106331.
Chen M, Chang YY, Huang S, Xiao LH, Zhou W, Zhang LY, et al. Aromatic-turmerone attenuates LPS-induced neuroinflammation and consequent memory impairment by targeting TLR4-dependent signaling pathway. Mol Nutr Food Res. 2018;62:201700281.
Gu SM, Park MH, Hwang CJ, Song HS, Lee US, Han SB, et al. Bee venom ameliorates lipopolysaccharide-induced memory loss by preventing NF-kappaB pathway. J Neuroinflamm. 2015;12:124.
Acknowledgements
This work was supported by Guangzhou Science Technology and Innovation Commission Technology Research Projects (201805010005), the National Natural Science Foundation of China (81473740, 81673627, 81673946, 81802776), the National Science Fund for Distinguished Young Scholars (81525026), and the National Key Research and Development Program of China “Research and Development of Comprehensive Technologies on Chemical Fertilizer and Pesticide Reduction and Synergism” (2017YFD0201402).
Author contributions
YLZ, YMY, HFP, YXC and YQL designed the experiments, collected and analyzed the data, and drafted the paper. YLZ, SYL and DHH performed the cellular experiments, YLZ, SYL and DHH performed the molecular experiments, and YLZ, SX, SFW, WL and YQL participated in animal surgery and data collection. YLZ, QW, LH, HFP, YXC and YQL revised the paper. All authors have read and approved the final submitted paper.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhou, Yl., Yan, Ym., Li, Sy. et al. 6-O-angeloylplenolin exerts neuroprotection against lipopolysaccharide-induced neuroinflammation in vitro and in vivo. Acta Pharmacol Sin 41, 10–21 (2020). https://doi.org/10.1038/s41401-019-0261-5
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-019-0261-5
Keywords
This article is cited by
-
MIF downregulation attenuates neuroinflammation via TLR4/MyD88/TRAF6/NF-κB pathway to protect dopaminergic neurons in Parkinson’s disease model
Communications Biology (2025)
-
Mechanistic prediction and validation of Brevilin A Therapeutic effects in Lung Cancer
BMC Complementary Medicine and Therapies (2024)
-
Isolation and anti-neuroinflammation activity of sesquiterpenoids from Artemisia argyi: computational simulation and experimental verification
BMC Complementary Medicine and Therapies (2024)
-
Advances in plant pathogen detection: integrating recombinase polymerase amplification with CRISPR/Cas systems
3 Biotech (2024)
-
Pharmacological Activities of Brevilin A: A Mini-Review
Current Pharmacology Reports (2024)