Abstract
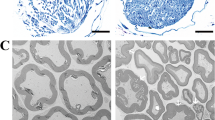
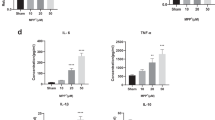
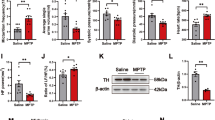
Parkinson’s disease (PD) is a multifactorial disorder characterized by progressive loss of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNpc) and the presence of Lewy bodies (LBs) consisting of misfolded α-synuclein protein. The etiology of PD is still not clear but systemic inflammation is proved to trigger and exacerbate DA neurons degeneration. Toll-like receptor 4 (TLR4) is a pattern-recognition receptor (PRR) and plays a major role in promoting the host immune. TLR4-mediated signal pathways induce the release of many inflammatory cytokines. It is reasonable to hypothesize that TLR4 is the mediator in microglia contributing to the damage of DA neurons in the SNpc. In this study, we evaluated the role of TLR4 in the chronic 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)/probenecid mouse model. Both TLR4-deficient and wild-type (WT) mice were injected with probenecid (250 mg/kg, i.p.) followed by injection of MPTP (25 mg/kg, s.c.) every 4 days for 10 times. From D43 to D47, the behavioral performance in pole test and wire hang test was assessed. Then the mice were euthanized, and SN and striatum were dissected out for biochemical tests. We showed that compared with MPTP-treated WT mice, TLR4 deficiency significantly attenuated MPTP-induced motor deficits and TH-protein expression reduction in SNpc and striatum, suppressed MPTP-induced α-synuclein abnormality and neuroinflammation mediated through oxidative stress, glial activation, NF-κB and the NLRP3 inflammasome signaling pathways. These findings highlight the neuroprotective effect of TLR4-pathways in the chronic MPTP-induced PD mouse model.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Erro R, Stamelou M. The motor syndrome of Parkinson’s disease. Int Rev Neurobiol. 2017;132:25–32.
Zeng XS, Geng WS, Jia JJ, Chen L, Zhang PP. Cellular and molecular basis of neurodegeneration in Parkinson disease. Front Aging Neurosci. 2018;10:109.
Vivekanantham S, Shah S, Dewji R, Dewji A, Khatri C, Ologunde R. Neuroinflammation in Parkinson’s disease: role in neurodegeneration and tissue repair. Int J Neurosci. 2015;125:717–25.
Kay DM, Factor SA, Samii A, Higgins DS, Griffith A, Roberts JW, et al. Genetic association between alpha-synuclein and idiopathic Parkinson’s disease. Am J Med Genet B Neuropsychiatr Genet. 2008;147b:1222–30.
Grozdanov V, Danzer KM. Release and uptake of pathologic alpha-synuclein. Cell Tissue Res. 2018;373:175–82.
Bridi JC, Hirth F. Mechanisms of alpha-Synuclein induced synaptopathy in Parkinson’s disease. Front Neurosci. 2018;12:80.
Lema Tome CM, Tyson T, Rey NL, Grathwohl S, Britschgi M, Brundin P. Inflammation and alpha-synuclein’s prion-like behavior in Parkinson’s disease-is there a link? Mol Neurobiol. 2013;47:561–74.
Beraud D, Maguire-Zeiss KA. Misfolded alpha-synuclein and toll-like receptors: therapeutic targets for Parkinson’s disease. Park Relat Disord. 2012;18(Suppl 1):S17–20.
Aloisi F. Immune function of microglia. Glia. 2001;36:165–79.
Drouin-Ouellet J, St-Amour I, Saint-Pierre M, Lamontagne-Proulx J, Kriz J, Barker RA, et al. Toll-like receptor expression in the blood and brain of patients and a mouse model of Parkinson’s disease. Int J Neuropsychopharmacol. 2014;18: pii: pyu103. https://doi.org/10.1093/ijnp/pyu103.
Noelker C, Morel L, Lescot T, Osterloh A, Alvarez-Fischer D, Breloer M, et al. Toll like receptor 4 mediates cell death in a mouse MPTP model of Parkinson disease. Sci Rep. 2013;3:1393.
Chen Y, Zhang QS, Shao QH, Wang S, Yuan YH, Chen NH, et al. NLRP3 inflammasome pathway is involved in olfactory bulb pathological alteration induced by MPTP. Acta Pharmacol Sin. 2019; 40: (in press). https://doi.org/10.1038/s41401-018-0209-1. [Epub ahead of print]
Zhang QS, Heng Y, Chen Y, Luo P, Wen L, Zhang Z, et al. A novel bibenzyl compound (20C) protects mice from 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/probenecid toxicity by regulating the alpha-synuclein-related inflammatory response. J Pharmacol Exp Ther. 2017;363:284–92.
Shaftel SS, Griffin WS, O’Banion MK. The role of interleukin-1 in neuroinflammation and Alzheimer disease: an evolving perspective. J Neuroinflammation. 2008;5:7.
Shao QH, Zhang XL, Yang PF, Yuan YH, Chen NH. Amyloidogenic proteins associated with neurodegenerative diseases activate the NLRP3 inflammasome. Int Immunopharmacol. 2017;49:155–60.
Sollberger G, Strittmatter GE, Garstkiewicz M, Sand J, Beer HD. Caspase-1: the inflammasome and beyond. Innate Immun. 2014;20:115–25.
Gustot A, Gallea JI, Sarroukh R, Celej MS, Ruysschaert JM, Raussens V. Amyloid fibrils are the molecular trigger of inflammation in Parkinson’s disease. Biochem J. 2015;471:323–33.
Tristao FS, Amar M, Latrous I, Del-Bel EA, Prediger RD, Raisman-Vozari R. Evaluation of nigrostriatal neurodegeneration and neuroinflammation following repeated intranasal 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) administration in mice, an experimental model of Parkinson’s disease. Neurotox Res. 2014;25:24–32.
Wu KC, Liou HH, Lee CY, Lin CJ. Down-regulation of natural resistance-associated macrophage protein-1 (Nramp1) is associated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)/1-methyl-4-phenylpyridinium (MPP( + ))-induced alpha-synuclein accumulation and neurotoxicity. Neuropathol Appl Neurobiol. 2019;45:157–73.
Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat Protoc. 2007;2:141–51.
Petroske E, Meredith GE, Callen S, Totterdell S, Lau YS. Mouse model of Parkinsonism: a comparison between subacute MPTP and chronic MPTP/probenecid treatment. Neuroscience. 2001;106:589–601.
Olivan S, Calvo AC, Rando A, Munoz MJ, Zaragoza P, Osta R. Comparative study of behavioural tests in the SOD1G93A mouse model of amyotrophic lateral sclerosis. Exp Anim. 2015;64:147–53.
Kovacs GG, Wagner U, Dumont B, Pikkarainen M, Osman AA, Streichenberger N, et al. An antibody with high reactivity for disease-associated alpha-synuclein reveals extensive brain pathology. Acta Neuropathol. 2012;124:37–50.
Unterberger U, Lachmann I, Voigtlander T, Pirker W, Berghoff AS, Flach K, et al. Detection of disease-associated alpha-synuclein in the cerebrospinal fluid: a feasibility study. Clin Neuropathol. 2014;33:329–34.
Maetzler W, Pilotto A, Apel A, Deuschle C, Kuebart G, Heinzel S, et al. In vivo markers of Parkinson’s disease and dementia with Lewy bodies: current value of the 5G4 alpha-synuclein antibody. Acta Neuropathol. 2014;128:893–5.
Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801.
Panaro MA, Lofrumento DD, Saponaro C, De Nuccio F, Cianciulli A, Mitolo V, et al. Expression of TLR4 and CD14 in the central nervous system (CNS) in a MPTP mouse model of Parkinson’s-like disease. Immunopharmacol Immunotoxicol. 2008;30:729–40.
Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, et al. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A. 2003;100:8514–9.
Shao QH, Yan WF, Zhang Z, Ma KL, Peng SY, Cao YL, et al. Nurr1: A vital participant in the TLR4-NF-kappaB signal pathway stimulated by alpha-synuclein in BV-2 cells. Neuropharmacology. 2019;144:388–99.
Jiang H, Wang Y, Liang X, Xing X, Xu X, Zhou C. Toll-like receptor 4 knockdown attenuates brain damage and neuroinflammation after traumatic brain injury via inhibiting neuronal autophagy and astrocyte activation. Cell Mol Neurobiol. 2018;38:1009–19.
Hoffmann A, Ettle B, Bruno A, Kulinich A, Hoffmann AC, von Wittgenstein J, et al. Alpha-synuclein activates BV2 microglia dependent on its aggregation state. Biochem Biophys Res Commun. 2016;479:881–6.
Malek N, Swallow D, Grosset KA, Anichtchik O, Spillantini M, Grosset DG. Alpha-synuclein in peripheral tissues and body fluids as a biomarker for Parkinson’s disease-a systematic review. Acta Neurol Scand. 2014;130:59–72.
Winner B, Jappelli R, Maji SK, Desplats PA, Boyer L, Aigner S, et al. In vivo demonstration that alpha-synuclein oligomers are toxic. Proc Natl Acad Sci U S A. 2011;108:4194–9.
Norris EH, Giasson BI, Ischiropoulos H, Lee VM. Effects of oxidative and nitrative challenges on alpha-synuclein fibrillogenesis involve distinct mechanisms of protein modifications. J Biol Chem. 2003;278:27230–40.
Xu Y, Deng Y, Qing H. The phosphorylation of alpha-synuclein: development and implication for the mechanism and therapy of the Parkinson’s disease. J Neurochem. 2015;135:4–18.
Dzamko N, Geczy CL, Halliday GM. Inflammation is genetically implicated in Parkinson’s disease. Neuroscience. 2015;302:89–102.
Stojkovska I, Wagner BM, Morrison BE. Parkinson’s disease and enhanced inflammatory response. Exp Biol Med (Maywood). 2015;240:1387–95.
Dursun E, Gezen-Ak D, Hanagasi H, Bilgic B, Lohmann E, Ertan S, et al. The interleukin 1 alpha, interleukin 1 beta, interleukin 6 and alpha-2-macroglobulin serum levels in patients with early or late onset Alzheimer’s disease, mild cognitive impairment or Parkinson’s disease. J Neuroimmunol. 2015;283:50–7.
Qiao C, Zhang Q, Jiang Q, Zhang T, Chen M, Fan Y, et al. Inhibition of the hepatic Nlrp3 protects dopaminergic neurons via attenuating systemic inflammation in a MPTP/p mouse model of Parkinson’s disease. J Neuroinflamm. 2018;15:193.
Qu J, Tao XY, Teng P, Zhang Y, Guo CL, Hu L, et al. Blocking ATP-sensitive potassium channel alleviates morphine tolerance by inhibiting HSP70-TLR4-NLRP3-mediated neuroinflammation. J Neuroinflamm. 2017;14:228.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81573640 and 81773925), the Beijing Natural Science Foundation (7161011), and the Drug Innovation Major Project 2018ZX09711-001-001 and 2018ZX09711-001-001-003.
Author information
Authors and Affiliations
Contributions
YHY and NHC designed research; QHS performed research; FFL, SW and XLZ contributed new analytical tools and reagents; YC analyzed data; QHS wrote the paper. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Shao, Qh., Chen, Y., Li, Ff. et al. TLR4 deficiency has a protective effect in the MPTP/probenecid mouse model of Parkinson’s disease. Acta Pharmacol Sin 40, 1503–1512 (2019). https://doi.org/10.1038/s41401-019-0280-2
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-019-0280-2
Keywords
This article is cited by
-
Deficient AMPK-SENP1-Sirt3 signaling impairs mitochondrial complex I function in Parkinson’s disease model
Translational Neurodegeneration (2025)
-
Inflammasomes in neurodegenerative diseases
Translational Neurodegeneration (2024)
-
Inflammasomes in neurological disorders — mechanisms and therapeutic potential
Nature Reviews Neurology (2024)
-
RGS5 augments astrocyte activation and facilitates neuroinflammation via TNF signaling
Journal of Neuroinflammation (2023)
-
Vancomycin Pretreatment on MPTP-Induced Parkinson’s Disease Mice Exerts Neuroprotection by Suppressing Inflammation Both in Brain and Gut
Journal of Neuroimmune Pharmacology (2023)