Abstract
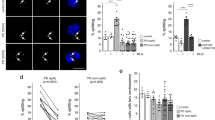
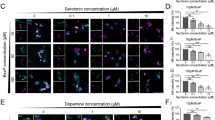
Stem cell therapy represents the potential alternative effective strategy for some diseases that lack effective treatment currently. Correspondingly, it is crucial to establish high-sensitive and reliable quantification assay for tracing exogenous cell migration. In the present study, we first used both bioluminescence imaging (BLI) indirect labeling (human norepinephrine transporter-luciferase reporter system) and 89zirconium (89Zr)-hNSCs direct labeling combined with positron emission tomography/computer tomography (PET/CT) system for tracking human neural stem cells (hNSCs) migration into the brain via nasal administration in preclinical study. But the above two methods failed to give the biodistribution profile due to their low sensitivity. Considering its superior sensitivity and absolute quantitation capability, we developed and validated the droplet digital PCR (ddPCR) targeting species-specific gene in frozen and paraffin sections, slices, and whole blood with the sensitivity of 100–200 hNSCs. Accurate and high throughput quantification could be performed using ddPCR with the coefficient of variation (CVs) of lower quality control (LQC) below 30%. In combination with immunohistochemistry and ddPCR, we confirmed the migration of hNSCs into the brain via nasal administration, which supported the efficacy of hNSCs in MPTP-treated mice, an animal model of Parkinson’s disease. In conclusion, the present study is the first to report the application of ddPCR in the pharmacokinetics profile description of tracking of hNSCs in preclinical studies.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ramos-Gómez M, Martínez-Serrano A. Tracking of iron-labeled human neural stem cells by magnetic resonance imaging in cellreplacement therapy for Parkinson’s disease. Neural Regen Res. 2016;11:49–52.
Liau MT, Amini F, Ramasamy TS. The therapeutic potential of stem cells and progenitor cells for the treatment of Parkinson’s disease. Tissue Eng Regen Med. 2016;13:455–64.
Liu Z, Li Z. Molecular imaging in tracking tumor-specific cytotoxic T lymphocytes (CTLs). Theranostics. 2014;4:990–1001.
Andrzejewska A, Nowakowski A, Janowski M, Bulte JW, Gilad AA, Walczak P. et al. Pre- and postmortem imaging of transplanted cells. Int J Nanomed. 2015;10:5543–59.
Gaudet JM, Ribot EJ, Chen Y, Gilbert KM, Foster PJ. Tracking the fate of stem cell implants with fluorine-19 MRI. PLoS One. 2015;10:e0118544.
Asiedu KO, Koyasu S, Szajek LP, Choyke PL, Sato N. Bone marrow cell trafficking analyzed by 89Zr-oxine positron emission tomography in a murine transplantation model. Clin Cancer Res. 2017;23:2759–68.
Moroz MA, Zhang H, Lee J, Moroz E, Zurita J, Shenke L, et al. Comparative analysis of T cell imaging with human nuclear reporter genes. J Nucl Med. 2015;56:1055–60.
Kallur T, Farr TD, Bohm-Sturm P, Kokaia Z, Hoehn M. Spatio-temporal dynamics, differentiation and viability of human neural stem cells after implantation into neonatal rat brain. Eur J Neurosci. 2011;34:382–93.
Song G, Chen M, Zhang Y, Cui L, Qu H, Zheng X, et al. Janus iron oxides@ semiconducting polymer nanoparticle tracer for cell tracking by magnetic particle imaging. Nano Lett. 2018;18:182–9.
Zheng B, Yu E, Orendorff R, Lu K, Konkle JJ, Tay ZW, et al. Seeing SPIOs directly in vivo with magnetic particle imaging. Mol Imaging Biol. 2017;19:385–90.
Nejadnik H, Pandit P, Lenkov O, Lahiji AP, Yerneni K, Daldrup-Link HE, et al. Ferumoxytol can be used for quantitative magnetic particle imaging of transplanted stem cells. Mol Imaging Biol. 2019;21:465–72.
Zheng B, von See MP, Yu E, Gunel B, Lu K, Vazin T, et al. Quantitative magnetic particle imaging monitors the transplantation, biodistribution, and clearance of stem cells in vivo. Theranostics. 2016;6:291–301.
Bossolasco P, Cova L, Levandis G, Diana V, Cerri S, Lambertenghi Deliliers G, et al. Noninvasive near-infrared live imaging of human adult mesenchymal stem cells transplanted in a rodent model of Parkinson's disease. Int J Nanomed. 2012;7:435–47.
Ramos-Gomez M, Seiz EG, Martinez-Serrano A. Optimization of the magnetic labeling of human neural stem cells and MRI visualization in the hemiparkinsonian rat brain. J Nanobiotechnol. 2015;13:20.
Eamegdool SS, Weible MW 2nd, Pham BT, Hawkett BS, Grieve SM, Chan-ling T, et al. Ultrasmall superparamagnetic iron oxide nanoparticle prelabeling of human neural precursor cells. Biomaterials. 2014;35:5549–64.
Schintu N, Frau L, Ibba M, Garau A, Carboni E, Carta AR, et al. Progressive dopaminergic degeneration in the chronic MPTP mouse model of Parkinson's disease. Neurotox Res.2009;16:127–39.
Quan PL, Sauzade M, Brouzes E. dPCR: a technology review. Sens (Basel). 2018;18:1271–98.
Bernsen MR, Guenoun J, van Tiel ST, Krestin GP. Nanoparticles and clinically applicable cell tracking. Br J Radiol. 2015;88:20150375.
Gonzalez R, Garitaonandia I, Poustovoitov M, Abramihina T, McEntire C, Culp B, et al. Neural stem cell derived from human parthenogenetic stem cells engraft and promote recovery in a nonhuman primate model of Parkinson's disease. Cell Transplant. 2016;25:1945–66.
Li X, Yang Q, Bai J, Xuan Y, Wang Y. Identification of appropriate reference genes for human mesenchymal stem cell analysis by quantitative real-time PCR. Biotechnol Lett. 2015;37:67–73.
China Food and Drug Administration. Technical Guidelines for the Development and Evaluation of Biosimilar Drugs (Trial). Beijing: China Food and Drug Administration; 2015.
Guidance for industry bioanalytical method validation. Biopharmaceutics Revision 1. Rockville (MD): US Department of Health and Human Services FDA (CDER) and (CVM); 2013. Docket Number:FDA-2013-D-1020.
EMEA. Guideline on bioanalytical method validation. Amsterdam (Netherland): EMEA; 2012.
Biological sample quantitative analysis method validation guidelines. In : Pharmacopoeia of People's Republic of China. Part 3. Beijing: Medical Science Press; 2015.
Dooves S, van der Knaap MS, Heine VM. Stem cell therapy for white matter disorders: don’t forget the microenvironment! J Inherit Metab Dis. 2016;39:513–8.
Abati E, Bresolin N, Comi GP, Corti S. Preconditioning and cellular engineering to increase the survival of transplanted neural stem cells for motor neuron disease therapy. Mol Neurobiol. 2019;56:3356–67.
Gao L, Xu W, Li T, Chen J, Shao A, Yan F, et al. Stem cell therapy: a promising therapeutic method for intracerebral hemorrhage. Cell Transplant. 2018;27:1809–24.
Zuo F, Xiong F, Wang X, Li X, Wang R, Ge W, et al. Intrastriatal transplantation of human neural stem cells restores the impaired subventricular zone in Parkinsonian mice. Stem Cells. 2017;35:1519–31.
Acknowledgements
This study was supported by the National Thirteen Five Major Special Fund “Nonclinical and Clinical PK, PD, and Immunogenicity Evaluation of Biosimilar” (2015ZX09501008-006) and “Core Technology-based Nonclinical Evaluation of Biological macromolecular drugs” (2015ZX09501007-002).
Author information
Authors and Affiliations
Contributions
JF, ZW and HFS designed the research; FW, ZW, FW, JZ, YJS, CFL, MJX, XC, SW, JWZ, XFZ, and XMW performed the research; FW, FW, KD, JZ, and JF analyzed the data; and ZW, FW, KD, JF, and HFS wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, F., Wang, Z., Wang, F. et al. Comparative strategies for stem cell biodistribution in a preclinical study. Acta Pharmacol Sin 41, 572–580 (2020). https://doi.org/10.1038/s41401-019-0313-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-019-0313-x
This article is cited by
-
Human iPSC-derived spinal neural progenitors enhance sensorimotor recovery in spinal cord-injured NOD-SCID mice via differentiation and microenvironment regulation
Cell Death & Disease (2025)
-
Mitochondrial DNA as a target for analyzing the biodistribution of cell therapy products
Scientific Reports (2024)
-
Advances in intranasal application of stem cells in the treatment of central nervous system diseases
Stem Cell Research & Therapy (2021)
-
Feasibility study of 68Ga-labeled CAR T cells for in vivo tracking using micro-positron emission tomography imaging
Acta Pharmacologica Sinica (2021)