Abstract
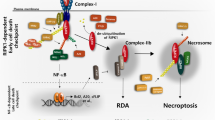
Receptor-interacting protein 1 (RIP1, also known as RIPK1) is not only a tumor-promoting factor in several cancers but also mediates either apoptosis or necroptosis in certain circumstances. In this study we investigated what role RIP1 plays in human ovarian cancer cells. We showed that knockout (KO) of RIP1 substantially suppressed cell proliferation, accompanied by the G2/M checkpoint arrest in two human ovarian cancer cell lines SKOV3 and A2780. On the other hand, RIP1 KO remarkably attenuated cisplatin-induced cytotoxicity, which was associated with reduction of the apoptosis markers PARP cleavage and the necroptosis marker phospho-MLKL. We found that RIP1 KO suppressed cisplatin-induced ROS accumulation in both SKOV3 and A2780 cells. ROS scavenger BHA, apoptosis inhibitor Z-VAD or necroptosis inhibitor NSA could effectively suppress cisplatin’s cytotoxicity in the control cells, suggesting that ROS-mediated apoptosis and necroptosis were involved in cisplatin-induced cell death. In addition, blocking necroptosis with MLKL siRNA effectively attenuated cisplatin-induced cytotoxicity. In human ovarian cancer A2780 cell line xenograft nude mice, RIP1 KO not only significantly suppressed the tumor growth but also greatly attenuated cisplatin’s anticancer activity. Our results demonstrate a dual role of RIP1 in human ovarian cancer: it acts as either a tumor-promoting factor to promote cancer cell proliferation or a tumor-suppressing factor to facilitate anticancer effects of chemotherapeutics such as cisplatin.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Kroeger PT Jr, Drapkin R. Pathogenesis and heterogeneity of ovarian cancer. Curr Opin Obstet Gynecol. 2017;29:26–34.
Testa U, Petrucci E, Pasquini L, Castelli G, Pelosi E. Ovarian cancers: genetic abnormalities, tumor heterogeneity and progression, clonal evolution and cancer stem cells. Medicines (Basel). 2018;5:E16.
La Vecchia C. Ovarian cancer: epidemiology and risk factors. Eur J Cancer Prev. 2017;26:55–62.
Festjens N, Vanden Berghe T, Cornelis S, Vandenabeele P. RIP1, a kinase on the crossroads of a cell's decision to live or die. Cell Death Differ. 2007;14:400–10.
Meylan E, Burns K, Hofmann K, Blancheteau V, Martinon F, Kelliher M, et al. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat Immunol. 2004;5:503–7.
Zhang DW, Zheng M, Zhao J, Li YY, Huang Z, Li Z, et al. Multiple death pathways in TNF-treated fibroblasts: RIP3- and RIP1-dependent and independent routes. Cell Res. 2011;21:368–71.
Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity. 1998;8:297–303.
Ting AT, Pimentel-Muinos FX, Seed B. RIP mediates tumor necrosis factor receptor 1 activation of NF-kappaB but not Fas/APO-1-initiated apoptosis. EMBO J. 1996;15:6189–96.
Lin Y, Devin A, Cook A, Keane MM, Kelliher M, Lipkowitz S, et al. The death domain kinase RIP is essential for TRAIL (Apo2L)-induced activation of IkappaB kinase and c-Jun N-terminal kinase. Mol Cell Biol. 2000;20:6638–45.
Kaiser WJ, Offermann MK. Apoptosis induced by the toll-like receptor adaptor TRIF is dependent on its receptor interacting protein homotypic interaction motif. J Immunol. 2005;174:4942–52.
Meylan E, Tschopp J. The RIP kinases: crucial integrators of cellular stress. Trends Biochem Sci. 2005;30:151–9.
Hur GM, Lewis J, Yang Q, Lin Y, Nakano H, Nedospasov S, et al. The death domain kinase RIP has an essential role in DNA damage-induced NF-kappa B activation. Genes Dev. 2003;17:873–82.
Park J, Kanayama A, Yamamoto K, Miyamoto Y. ARD1 binding to RIP1 mediates doxorubicin-induced NF-kappaB activation. Biochem Biophys Res Commun. 2012;422:291–7.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Bai L, Xu S, Chen W, Li Z, Wang X, Tang H, et al. Blocking NF-kappaB and Akt by Hsp90 inhibition sensitizes Smac mimetic compound 3-induced extrinsic apoptosis pathway and results in synergistic cancer cell death. Apoptosis. 2011;16:45–54.
Palacios C, Lopez-Perez AI, Lopez-Rivas A. Down-regulation of RIP expression by 17-dimethylaminoethylamino-17-demethoxygeldanamycin promotes TRAIL-induced apoptosis in breast tumor cells. Cancer Lett. 2010;287:207–15.
Wang X, Ju W, Renouard J, Aden J, Belinsky SA, Lin Y. 17-allylamino-17-demethoxygeldanamycin synergistically potentiates tumor necrosis factor-induced lung cancer cell death by blocking the nuclear factor-kappaB pathway. Cancer Res. 2006;66:1089–95.
Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703.
Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–95.
Lin Y, Choksi S, Shen HM, Yang QF, Hur GM, Kim YS, et al. Tumor necrosis factor-induced nonapoptotic cell death requires receptor-interacting protein-mediated cellular reactive oxygen species accumulation. J Biol Chem. 2004;279:10822–8.
Tenev T, Bianchi K, Darding M, Broemer M, Langlais C, Wallberg F, et al. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol Cell. 2011;43:432–48.
O'Donnell MA, Ting AT. RIP1 comes back to life as a cell death regulator in TNFR1 signaling. FEBS J. 2011;278:877–87.
Degterev A, Ofengeim D, Yuan J. Targeting RIPK1 for the treatment of human diseases. Proc Natl Acad Sci USA. 2019;116:9714–22.
Kondylis V, Pasparakis M. RIP kinases in liver cell death, inflammation and cancer. Trends Mol Med. 2019;25:47–63.
Park S, Hatanpaa KJ, Xie Y, Mickey BE, Madden CJ, Raisanen JM, et al. The receptor interacting protein 1 inhibits p53 induction through NF-kappaB activation and confers a worse prognosis in glioblastoma. Cancer Res. 2009;69:2809–16.
Wang Q, Chen W, Xu X, Li B, He W, Padilla MT, et al. RIP1 potentiates BPDE-induced transformation in human bronchial epithelial cells through catalase-mediated suppression of excessive reactive oxygen species. Carcinogenesis. 2013;34:2119–28.
Liu XY, Lai F, Yan XG, Jiang CC, Guo ST, Wang CY, et al. RIP1 kinase is an oncogenic driver in melanoma. Cancer Res. 2015;75:1736–48.
Wang Q, Chen W, Bai L, Chen W, Padilla MT, Lin AS, et al. Receptor-interacting protein 1 increases chemoresistance by maintaining inhibitor of apoptosis protein levels and reducing reactive oxygen species through a microRNA-146a-mediated catalase pathway. J Biol Chem. 2014;289:5654–63.
Wang R, Zheng X, Zhang L, Zhou B, Hu H, Li Z, et al. Histone H4 expression is cooperatively maintained by IKKbeta and Akt1 which attenuates cisplatin-induced apoptosis through the DNA-PK/RIP1/IAPs signaling cascade. Sci Rep. 2017;7:41715.
Wang Q, Shi S, He W, Padilla MT, Zhang L, Wang X, et al. Retaining MKP1 expression and attenuating JNK-mediated apoptosis by RIP1 for cisplatin resistance through miR-940 inhibition. Oncotarget. 2014;5:1304–14.
Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19:107–20.
Galluzzi L, Aaronson SA, Abrams J, Alnemri ES, Andrews DW, Baehrecke EH, et al. Guidelines for the use and interpretation of assays for monitoring cell death in higher eukaryotes. Cell Death Differ. 2009;16:1093–107.
He S, Huang S, Shen Z. Biomarkers for the detection of necroptosis. Cell Mol Life Sci. 2016;73:2177–81.
Lin Y, Devin A, Rodriguez Y, Liu ZG. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13:2514–26.
Lei FX, Jin L, Liu XY, Lai F, Yan XG, Farrelly M, et al. RIP1 protects melanoma cells from apoptosis induced by BRAF/MEK inhibitors. Cell Death Dis. 2018;9:679.
Lin Y, Yang Q, Wang X, Liu ZG. The essential role of the death domain kinase receptor-interacting protein in insulin growth factor-I-induced c-Jun N-terminal kinase activation. J Biol Chem. 2006;281:23525–32.
Chen W, Wang Q, Bai L, Chen W, Wang X, Tellez CS, et al. RIP1 maintains DNA integrity and cell proliferation by regulating PGC-1alpha-mediated mitochondrial oxidative phosphorylation and glycolysis. Cell Death Differ. 2014;21:1061–70.
Chauhan AK, Min KJ, Kwon TK. RIP1-dependent reactive oxygen species production executes artesunate-induced cell death in renal carcinoma Caki cells. Mol Cell Biochem. 2017;435:15–24.
Darding M, Meier P. IAPs: guardians of RIPK1. Cell Death Differ. 2012;19:58–66.
Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ, et al. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135:1311–23.
Zhou W, Yuan J. Necroptosis in health and diseases. Semin Cell Dev Biol. 2014;35:14–23.
Bertrand MJ, Vandenabeele P. The Ripoptosome: death decision in the cytosol. Mol Cell. 2011;43:323–5.
Galluzzi L, Vanden Berghe T, Vanlangenakker N, Buettner S, Eisenberg T, Vandenabeele P, et al. Programmed necrosis from molecules to health and disease. Int Rev Cell Mol Biol. 2012;289:1–35.
He W, Wang Q, Srinivasan B, Xu J, Padilla MT, Li Z, et al. A JNK-mediated autophagy pathway that triggers c-IAP degradation and necroptosis for anticancer chemotherapy. Oncogene. 2014;33:3004–13.
Xu Y, Ma HB, Fang YL, Zhang ZR, Shao J, Hong M, et al. Cisplatin-induced necroptosis in TNFalpha dependent and independent pathways. Cell Signal. 2017;31:112–23.
Acknowledgements
This work was supported in part by grants from the National Natural Science Foundation of China (Numbers 81172494 and 81670346), the R&D Program for International S&T Cooperation and the Exchanges of Sichuan province (Number 2018HH0014), and NCI/NIH (1R21CA193633).
Author information
Authors and Affiliations
Contributions
BZ and YL designed the research and wrote the manuscript. XLZ, JJY, YYW, QL, YPS and MS performed the experiments and analyzed the data. JKL, LZ and ZPL participated in the research design and manuscript preparation. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zheng, Xl., Yang, Jj., Wang, Yy. et al. RIP1 promotes proliferation through G2/M checkpoint progression and mediates cisplatin-induced apoptosis and necroptosis in human ovarian cancer cells. Acta Pharmacol Sin 41, 1223–1233 (2020). https://doi.org/10.1038/s41401-019-0340-7
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-019-0340-7
Keywords
This article is cited by
-
Interplay between NETosis and the lncRNA-microRNA regulatory axis in the immunopathogenesis of cancer
Journal of Physiology and Biochemistry (2025)
-
Developing a prognostic model for hepatocellular carcinoma based on MED19 and clinical stage and determining MED19 as a therapeutic target
Journal of Cancer Research and Clinical Oncology (2024)
-
Downregulation of Linc00173 increases BCL2 mRNA stability via the miR-1275/PROCA1/ZFP36L2 axis and induces acquired cisplatin resistance of lung adenocarcinoma
Journal of Experimental & Clinical Cancer Research (2023)
-
A novel RIP1-mediated canonical WNT signaling pathway that promotes colorectal cancer metastasis via β -catenin stabilization-induced EMT
Cancer Gene Therapy (2023)
-
A comprehensive pan-cancer analysis of necroptosis molecules in four gynecologic cancers
BMC Cancer (2022)