Abstract
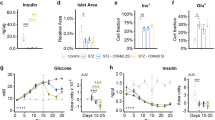
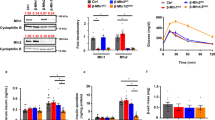
Mitochondria serve as sensors of energy regulation and glucose levels, which are impaired by diabetes progression. Catalpol is an iridoid glycoside that exerts a hypoglycemic effect by improving mitochondrial function, but the underlying mechanism has not been fully elucidated. In the current study we explored the effects of catalpol on mitochondrial function in db/db mice and C2C12 myotubes in vitro. After oral administration of catalpol (200 mg·kg−1·d−1) for 8 weeks, db/db mice exhibited a decreased fasting blood glucose level and restored mitochondrial function in skeletal muscle. Catalpol increased mitochondrial biogenesis, evidenced by significant elevations in the number of mitochondria, mitochondrial DNA levels, and the expression of three genes associated with mitochondrial biogenesis: peroxisome proliferator-activated receptor gammaco-activator 1 (PGC-1α), mitochondrial transcription factor A (TFAM) and nuclear respiratory factor 1 (NRF1). In C2C12 myotubes, catalpol significantly increased glucose uptake and ATP production. These effects depended on activation of AMP-activated protein kinase (AMPK)-mediated mitochondrial biogenesis. Thus, catalpol improves skeletal muscle mitochondrial function by activating AMPK-mediated mitochondrial biogenesis. These findings may guide the development of a new therapeutic approach for type 2 diabetes.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37:S81–90.
Oriente F, Iovino S, Cabaro S, Cassese A, Longobardi E, Miele C, et al. Prep1 controls insulin glucoregulatory function in liver by transcriptional targeting of SHP1 tyrosine phosphatase. Diabetes. 2011;60:138–47.
Zamora M, Pardo R, Villena JA. Pharmacological induction of mitochondrial biogenesis as a therapeutic strategy for the treatment of type 2 diabetes. Biochem Pharmacol. 2015;98:16–28.
Yaribeygi H, Atkin SL, Sahebkar A. Mitochondrial dysfunction in diabetes and the regulatory roles of antidiabetic agents on the mitochondrial function. J Cell Physiol. 2019;234:8402–10.
Rector RS, Thyfault JP, Uptergrove GM, Morris EM, Naples SP, Borengasser SJ, et al. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J Hepatol. 2010;52:727–36.
Hesselink MKC, Schrauwen-Hinderling V, Schrauwen P. Skeletal muscle mitochondria as a target to prevent or treat type 2 diabetes mellitus. Nat Rev Endocrinol. 2016;12:633–45.
Deng XH, Liu JJ, Sun XJ, Dong JC, Huang JH. Benzoylaconine induces mitochondrial biogenesis in mice via activating AMPK signaling cascade. Acta Pharmacol Sin. 2019;40:658–65.
Hancock ML, Meyer RC, Mistry M, Khetani RS, Wagschal A, Shin T, et al. Insulin receptor associates with promoters genome-wide and regulates gene expression. Cell. 2019;177:722–36.
Schrauwen-Hinderling VB, Kooi ME, Hesselink MK, Jeneson JA, Backes WH, van Echteld CJ, et al. Impaired in vivo mitochondrial function but similar intramyocellular lipid content in patients with type 2 diabetes mellitus and BMI-matched control subjects. Diabetologia. 2007;50:113–20.
Xu DQ, Jiang ZZ, Sun ZR, Wang L, Zhao GL, Hassan HM, et al. Mitochondrial dysfunction and inhibition of myoblast differentiation in mice with high-fat-diet-induced pre-diabetes. J Cell Physiol. 2019;234:7510–23.
Barreiro E, Puig-Vilanova E, Marin-Corral J, Chacon-Cabrera A, Salazar-Degracia A, Mateu X, et al. Therapeutic approaches in mitochondrial dysfunction, proteolysis, and structural alterations of diaphragm and gastrocnemius in rats with chronic heart failure. J Cell Physiol. 2016;231:1495–513.
Andreux PA, Houtkooper RH, Auwerx J. Pharmacological approaches to restore mitochondrial function. Nat Rev Drug Discov. 2013;12:465–83.
Befroy DE, Petersen KF, Dufour S, Mason GF, de Graaf RA, Rothman DL, et al. Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes. 2007;56:1376–81.
Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes. 2005;54:8–14.
Fang WJ, Wang CJ, He Y, Zhou YL, Peng XD, Liu SK. Resveratrol alleviates diabetic cardiomyopathy in rats by improving mitochondrial function through PGC1α deacetylation. Acta Pharmacol Sin. 2018;39:59–73.
Yan WJ, Zhang HF, Liu PL, Wang H, Liu JY, Gao C, et al. Impaired mitochondrial biogenesis due to dysfunctional adiponectin-AMPK-PGC-1α signaling contributing to increased vulnerability in diabetic heart. Basic Res Cardiol. 2013;108:329–43.
Whitaker RM, Corum D, Beeson CC, Schnellmann RG. Mitochondrial biogenesis as a pharmacological target: a new approach to acute and chronic diseases. Annu Rev Pharm Toxicol. 2016;56:229–49.
Villena JA. New insights into PGC-1 coactivators: redefining their role in the regulation of mitochondrial function and beyond. FEBS J. 2015;282:647–72.
Gleyzer N, Vercauteren K, Scarpulla RC. Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol Cell Biol. 2005;25:1354–66.
Yun CW, Han YS, Lee SH. PGC-1α controls mitochondrial biogenesis in drug-resistant colorectal cancer cells by regulating endoplasmic reticulum stress. Int J Mol Sci. 2019;20:1707–20.
Halling JF, Jessen H, Nohr-Meldgaard J, Thiellesen Buch B, Masselkhi Christensen N, Gudiksen A, et al. PGC-1α regulates mitochondrial properties beyond biogenesis with aging and exercise training. Am J Physiol Endocrinol Metab. 2019;317:E513–25.
Xu DQ, Wang L, Jiang ZZ, Zhao GL, Hassan HM, Sun LX, et al. A new hypoglycemic mechanism of catalpol revealed by enhancing MyoD/MyoG-mediated myogenesis. Life Sci. 2018;209:313–23.
Dong Z, Chen CX. Effect of catalpol on diabetic nephropathy in rats. Phytomedicine. 2013;20:1023–9.
Zhou J, Xu G, Ma S, Li F, Yuan M, Xu HB, et al. Catalpol ameliorates high-fat diet-induced insulin resistance and adipose tissue inflammation by suppressing the JNK and NF-kappaB pathways. Biochem Biophys Res Commun. 2015;467:853–8.
Li X, Xu ZM, Jiang ZZ, Sun LX, Ji JZ, Miao JS, et al. Hypoglycemic effect of catalpol on high-fat diet/streptozotocin-induced diabetic mice by increasing skeletal muscle mitochondrial biogenesis. Acta Biochim Biophys Sin (Shanghai). 2014;46:738–48.
Xu ZM, Zhang LY, Li XJ, Jiang ZZ, Sun LX, Zhao GL, et al. Mitochondrial fusion/fission process involved in the improvement of catalpol on high glucose-induced hepatic mitochondrial dysfunction. Acta Biochim Biophys Sin (Shanghai). 2015;47:730–40.
Yi JS, Park JS, Ham YM, Nguyen N, Lee NR, Hong J, et al. MG53-induced IRS-1 ubiquitination negatively regulates skeletal myogenesis and insulin signalling. Nat Commun 2013;4:2354–77.
Park SY, Kim MH, Ahn JH, Lee SJ, Lee JH, Eum WS, et al. The stimulatory effect of essential fatty acids on glucose uptake involves both Akt and AMPK activation in C2C12 skeletal muscle cells. Korean J Physiol Pharmacol. 2014;18:255–61.
Zhao GL, Xu DQ, Yuan ZQ, Jiang ZZ, Zhou W, Li ZJ, et al. 8-Methoxypsoralen disrupts MDR3-mediated phospholipids efflux and bile acid homeostasis and its relevance to hepatotoxicity. Toxicology 2017;386:40–8.
Kim JY, Choi MJ, So B, Kim HJ, Seong JK, Song W. The preventive effects of 8 weeks of resistance training on glucose tolerance and muscle fiber type composition in zucker rats. Diabetes Metab J. 2015;39:424–33.
Houstek J, Pickova A, Vojtiskova A, Mracek T, Pecina P, Jesina P. Mitochondrial diseases and genetic defects of ATP synthase. Biochim Biophys Acta. 2006;1757:1400–5.
Chen XL, Wei SS, Yang FQ. Mitochondria in the pathogenesis of diabetes: a proteomic view. Protein Cell. 2012;3:648–60.
Venojarvi M, Puhke R, Hamalainen H, Marniemi J, Rastas M, Rusko H, et al. Role of skeletal muscle-fibre type in regulation of glucose metabolism in middle-aged subjects with impaired glucose tolerance during a long-term exercise and dietary intervention. Diabetes Obes Metab. 2005;7:745–54.
Jager S, Handschin C, Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc Natl Acad Sci USA. 2007;104:12017–22.
Kabra UD, Pfuhlmann K, Migliorini A, Keipert S, Lamp D, Korsgren O, et al. Direct substrate delivery into mitochondrial fission-deficient pancreatic islets rescues insulin secretion. Diabetes. 2017;66:1247–57.
Chopra I, Li HF, Wang H, Webster KA. Phosphorylation of the insulin receptor by AMP-activated protein kinase (AMPK) promotes ligand-independent activation of the insulin signalling pathway in rodent muscle. Diabetologia 2012;55:783–94.
Bao QW, Shen XZ, Qian L, Gong C, Nie MX, Dong Y. Anti-diabetic activities of catalpol in db/db mice. Korean J Physiol Pharmacol. 2016;20:153–60.
Affourtit C. Mitochondrial involvement in skeletal muscle insulin resistance: a case of imbalanced bioenergetics. Biochim Biophys Acta. 2016;1857:1678–93.
Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–2.
Carling D. AMPK signalling in health and disease. Curr Opin Cell Biol. 2017;45:31–7.
Marin TL, Gongol B, Zhang F, Martin M, Johnson DA, Xiao H, et al. AMPK promotes mitochondrial biogenesis and function by phosphorylating the epigenetic factors DNMT1, RBBP7, and HAT1. Sci Signal. 2017;10:7478–98.
Misu H, Takayama H, Saito Y, Mita Y, Kikuchi A, Ishii K, et al. Deficiency of the hepatokine selenoprotein P increases responsiveness to exercise in mice through upregulation of reactive oxygen species and AMP-activated protein kinase in muscle. Nat Med. 2017;23:508–16.
Holloway GP. Mitochondrial function and dysfunction in exercise and insulin resistance. Appl Physiol Nutr Metab. 2009;34:440–6.
Wanagat J, Hevener AL. Mitochondrial quality control in insulin resistance and diabetes. Curr Opin Genet Dev. 2016;38:118–26.
Wu HJ, Deng XN, Shi YH, Su Y, Wei JY, Duan HJ. PGC-1α, glucose metabolism and type 2 diabetes mellitus. J Endocrinol. 2016;229:R99–115.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81403154, 81773827, 81573514, 81773995, 81873084, 81573690); the Scholar of the 14th Batch of “Six Talents Peak” High-level Talent Selection program (SWYY-094); the Postgraduate Research Practice Innovation Program of Jiangsu Province (KYCX19-0763); the “Double First-Class” University project (CPU2018GY33); the Qinghai Province High-End Innovative Thousand Talents Program; and the Tianshan Cedar Project of the Xinjiang Uygur Autonomous Region (2018XS21).
Author information
Authors and Affiliations
Contributions
DQX and LW analyzed the data, contributed to discussions and wrote the manuscript. DQX, CJL, and SSF collected the data. ZJL and HFH analyzed the data. LXS reviewed the manuscript. TW and ZZJ conceived the experiments, analyzed the data and edited/reviewed the manuscript. LYZ is the guarantor of this work, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Xu, Dq., Li, Cj., Jiang, Zz. et al. The hypoglycemic mechanism of catalpol involves increased AMPK-mediated mitochondrial biogenesis. Acta Pharmacol Sin 41, 791–799 (2020). https://doi.org/10.1038/s41401-019-0345-2
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-019-0345-2
Keywords
This article is cited by
-
Multifaceted therapeutic potentials of catalpol, an iridoid glycoside: an updated comprehensive review
Inflammopharmacology (2025)
-
A study on the mechanism of Beclin-1 m6A modification mediated by catalpol in protection against neuronal injury and autophagy following cerebral ischemia
Molecular Medicine (2024)
-
Mitochondrial quality control in human health and disease
Military Medical Research (2024)
-
The potential of therapeutic strategies targeting mitochondrial biogenesis for the treatment of insulin resistance and type 2 diabetes mellitus
Archives of Pharmacal Research (2024)
-
The role of Huidouba in regulating skeletal muscle metabolic disorders in prediabetic mice through AMPK/PGC-1α/PPARα pathway
Diabetology & Metabolic Syndrome (2023)