Abstract
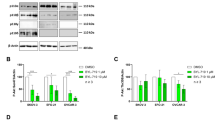
Anexelekto (AXL), a member of the TYRO3-AXL-MER (TAM) family of receptor tyrosine kinases (RTK), is overexpressed in varieties of tumor tissues and promotes tumor development by regulating cell proliferation, migration and invasion. In this study, we investigated the role of AXL in regulating glycolysis in human ovarian cancer (OvCa) cells. We showed that the expression of AXL mRNA and protein was significantly higher in OvCa tissue than that in normal ovarian epithelial tissue. In human OvCa cell lines suppression of AXL significantly inhibited cell proliferation, and increased the sensitivity of OvCa cells to cisplatin, which also proved by nude mice tumor formation experiment. KEGG analysis showed that AXL was significantly enriched in the glycolysis pathways of cancer. Changes in AXL expression in OvCa cells affect tumor glycolysis. We demonstrated that the promotion effect of AXL on glycolysis was mediated by phosphorylating the M2 isoform of pyruvate kinase (PKM2) at Y105. AXL expression was significantly higher in cisplatin-resistant OvCa cells A2780/DDP compared with the parental A2780 cells. Inhibition of AXL decreased the level of glycolysis in A2780/DDP cells, and increased the cytotoxicity of cisplatin against A2780/DDP cells, suggesting that AXL-mediated glycolysis was associated with cisplatin resistance in OvCa. In conclusion, this study demonstrates for the first time that AXL is involved in the regulation of the Warburg effect. Our results not only highlight the clinical value of targeting AXL, but also provide theoretical basis for the combination of AXL inhibitor and cisplatin in the treatment of OvCa.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: A Cancer J Clin. 2020;70:7–30.
Coleman RL, Monk BJ, Sood AK, Herzog TJ. Latest research and treatment of advanced-stage epithelial ovarian cancer. Nat Rev Clin Oncol. 2013;10:211–24.
Liu J, Matulonis UA. New strategies in ovarian cancer: translating the molecular complexity of ovarian cancer into treatment advances. Clin Cancer Res. 2014;20:5150–6.
Vaughan S, Coward JI, Bast RC Jr., Berchuck A, Berek JS, Brenton JD, et al. Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer. 2011;11:719–25.
De Giorgi U, Casadei C, Bergamini A, Attademo L, Cormio G, Lorusso D, et al. Therapeutic challenges for cisplatin-resistant ovarian germ cell tumors. Cancers (Basel). 2019;11:1584.
Lozneanu L, Pinciroli P, Ciobanu DA, Carcangiu ML, Canevari S, Tomassetti A, et al. Computational and immunohistochemical analyses highlight AXL as a potential prognostic marker for ovarian cancer patients. Anticancer Res. 2016;36:4155–63.
Zhu C, Wei Y, Wei X. AXL receptor tyrosine kinase as a promising anti-cancer approach: functions, molecular mechanisms and clinical applications. Mol Cancer. 2019;18:153.
Meyer AS, Miller MA, Gertler FB, Lauffenburger DA. The receptor AXL diversifies EGFR signaling and limits the response to EGFR-targeted inhibitors in triple-negative breast cancer cells. Sci Signal. 2013;6:ra66.
Okimoto RA, Bivona TG. AXL receptor tyrosine kinase as a therapeutic target in NSCLC. Lung Cancer (Auckl). 2015;6:27–34.
Tworkoski KA, Platt JT, Bacchiocchi A, Bosenberg M, Boggon TJ, Stern DF. MERTK controls melanoma cell migration and survival and differentially regulates cell behavior relative to AXL. Pigment Cell Melanoma Res. 2013;26:527–41.
Paccez JD, Vasques GJ, Correa RG, Vasconcellos JF, Duncan K, Gu X, et al. The receptor tyrosine kinase Axl is an essential regulator of prostate cancer proliferation and tumor growth and represents a new therapeutic target. Oncogene. 2013;32:689–98.
Myers SH, Brunton VG, Unciti-Broceta A. AXL inhibitors in cancer: a medicinal chemistry perspective. J Med Chem. 2016;59:3593–608.
Vouri M, Hafizi S. TAM receptor tyrosine kinases in cancer drug resistance. Cancer Res. 2017;77:2775–8.
Dunne PD, McArt DG, Blayney JK, Kalimutho M, Greer S, Wang T, et al. AXL is a key regulator of inherent and chemotherapy-induced invasion and predicts a poor clinical outcome in early-stage colon cancer. Clin Cancer Res. 2014;20:164–75.
Keating AK, Kim GK, Jones AE, Donson AM, Ware K, Mulcahy JM, et al. Inhibition of Mer and Axl receptor tyrosine kinases in astrocytoma cells leads to increased apoptosis and improved chemosensitivity. Mol Cancer Ther. 2010;9:1298–307.
Hong CC, Lay JD, Huang JS, Cheng AL, Tang JL, Lin MT, et al. Receptor tyrosine kinase AXL is induced by chemotherapy drugs and overexpression of AXL confers drug resistance in acute myeloid leukemia. Cancer Lett. 2008;268:314–24.
Hong J, Peng D, Chen Z, Sehdev V, Belkhiri A. ABL regulation by AXL promotes cisplatin resistance in esophageal cancer. Cancer Res. 2013;73:331–40.
Quinn JM, Greenwade MM, Palisoul ML, Opara G, Massad K, Guo L, et al. Therapeutic inhibition of the receptor tyrosine kinase AXL improves sensitivity to platinum and taxane in ovarian cancer. Mol Cancer Ther. 2019;18:389–98.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Warburg O. On the origin of cancer cells. Science. 1956;123:309–14.
Warburg O, Wind FEN. In metabolism of tumours. J Gen Physiol. 1927;8:519–30.
Bhattacharya B, Mohd Omar MF, Soong R. The Warburg effect and drug resistance. Br J Pharm. 2016;173:970–9.
Hua G, Liu Y, Li X, Xu P, Luo Y. Targeting glucose metabolism in chondrosarcoma cells enhances the sensitivity to doxorubicin through the inhibition of lactate dehydrogenase-A. Oncol Rep. 2014;31:2727–34.
Zhang X, Ai Z, Chen J, Yi J, Liu Z, Zhao H, et al. Glycometabolic adaptation mediates the insensitivity of drug-resistant K562/ADM leukaemia cells to adriamycin via the AKT-mTOR/c-Myc signalling pathway. Mol Med Rep. 2017;15:1869–76.
Zhou M, Zhao Y, Ding Y, Liu H, Liu Z, Fodstad O, et al. Warburg effect in chemosensitivity: targeting lactate dehydrogenase-A re-sensitizes taxol-resistant cancer cells to taxol. Mol Cancer. 2010;9:33.
Wiese EK, Hitosugi T. Tyrosine kinase signaling in cancer metabolism: PKM2 paradox in the Warburg effect. Front Cell Dev Biol. 2018;6:79.
Israelsen WJ, Dayton TL, Davidson SM, Fiske BP, Hosios AM, Bellinger G, et al. PKM2 isoform-specific deletion reveals a differential requirement for pyruvate kinase in tumor cells. Cell. 2013;155:397–409.
Chao TK, Huang TS, Liao YP, Huang RL, Su PH, Shen HY, et al. Pyruvate kinase M2 is a poor prognostic marker of and a therapeutic target in ovarian cancer. PLoS One. 2017;12:e0182166.
Talekar M, Ouyang Q, Goldberg MS, Amiji MM. Cosilencing of PKM-2 and MDR-1 sensitizes multidrug-resistant ovarian cancer cells to paclitaxel in a murine model of ovarian cancer. Mol Cancer Ther. 2015;14:1521–31.
Chen X, Wei S, Ma Y, Lu J, Niu G, Xue Y, et al. Quantitative proteomics analysis identifies mitochondria as therapeutic targets of multidrug-resistance in ovarian cancer. Theranostics. 2014;4:1164–75.
Christofk HR, Vander Heiden MG, Wu N, Asara JM, Cantley LC. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature. 2008;452:181–6.
Hitosugi T, Kang S, Vander Heiden MG, Chung TW, Elf S, Lythgoe K, et al. Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci Signal. 2009;2:ra73.
Li FL, Liu JP, Bao RX, Yan G, Feng X, Xu YP, et al. Acetylation accumulates PFKFB3 in cytoplasm to promote glycolysis and protects cells from cisplatin-induced apoptosis. Nat Commun. 2018;9:508.
Loar P, Wahl H, Kshirsagar M, Gossner G, Griffith K, Liu JR. Inhibition of glycolysis enhances cisplatin-induced apoptosis in ovarian cancer cells. Am J Obstet Gynecol. 2010;202:371 e1–8.
Fekete JánosT, Győrffy B. ROCplot.org: Validating predictive biomarkers of chemotherapy/hormonal therapy/anti-HER2 therapy using transcriptomic data of 3,104 breast cancer patients. Int J Cancer. 2019;145:3140–51.
Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384:1376–88.
Deborah K, Ronald D, Jamie N. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines), Ovarian Cancer. https://www.tri-kobe.org/ (2019).
Rankin EB, Giaccia AJ. The receptor tyrosine kinase AXL in cancer progression. Cancers (Basel). 2016;8:103.
Paccez JD, Duncan K, Vava A, Correa RG, Libermann TA, Parker MI, et al. Inactivation of GSK3beta and activation of NF-kappaB pathway via Axl represents an important mediator of tumorigenesis in esophageal squamous cell carcinoma. Mol Biol Cell. 2015;26:821–31.
Ou WB, Corson JM, Flynn DL, Lu WP, Wise SC, Bueno R, et al. AXL regulates mesothelioma proliferation and invasiveness. Oncogene. 2011;30:1643–52.
Sun W, Fujimoto J, Tamaya T. Coexpression of Gas6/Axl in human ovarian cancers. Oncology 2004;66:450–7.
US National Library of Medicine. ClinicalTrials.gov, <https://clinicaltrials.gov> (2019).
Linger RM, Cohen RA, Cummings CT, Sather S, Migdall-Wilson J, Middleton DH, et al. Mer or Axl receptor tyrosine kinase inhibition promotes apoptosis, blocks growth and enhances chemosensitivity of human non-small cell lung cancer. Oncogene. 2013;32:3420–31.
Myers KV, Amend SR, Pienta KJ. Targeting Tyro3, Axl and MerTK (TAM receptors): implications for macrophages in the tumor microenvironment. Mol Cancer. 2019;18:94.
Brand TM, Iida M, Stein AP, Corrigan KL, Braverman CM, Luthar N, et al. AXL mediates resistance to cetuximab therapy. Cancer Res. 2014;74:5152–64.
Miller MA, Oudin MJ, Sullivan RJ, Wang SJ, Meyer AS, Im H, et al. Reduced proteolytic shedding of receptor tyrosine kinases is a post-translational mechanism of kinase inhibitor resistance. Cancer Disco. 2016;6:382–99.
Konieczkowski DJ, Johannessen CM, Abudayyeh O, Kim JW, Cooper ZA, Piris A, et al. A melanoma cell state distinction influences sensitivity to MAPK pathway inhibitors. Cancer Disco. 2014;4:816–27.
Elkabets M, Pazarentzos E, Juric D, Sheng Q, Pelossof RA, Brook S, et al. AXL mediates resistance to PI3Kalpha inhibition by activating the EGFR/PKC/mTOR axis in head and neck and esophageal squamous cell carcinomas. Cancer Cell. 2015;27:533–46.
Wang C, Jin H, Wang N, Fan S, Wang Y, Zhang Y, et al. Gas6/Axl Axis contributes to chemoresistance and metastasis in breast cancer through Akt/GSK-3beta/beta-catenin signaling. Theranostics. 2016;6:1205–19.
Suh YA, Jo SY, Lee HY, Lee C. Inhibition of IL-6/STAT3 axis and targeting Axl and Tyro3 receptor tyrosine kinases by apigenin circumvent taxol resistance in ovarian cancer cells. Int J Oncol. 2015;46:1405–11.
Zhao Y, Butler EB, Tan M. Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis. 2013;4:532.
Zhou Y, Tozzi F, Chen J, Fan F, Xia L, Wang J, et al. Intracellular ATP levels are a pivotal determinant of chemoresistance in colon cancer cells. Cancer Res. 2012;72:304–14.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No 81972480), and the Postgraduate Research and Innovation Project of Central South University (grant number: 2019zzts 769). A2780/DDP and A2780 cells were gifted by Pingan Ma (State Key Laboratory of Rare Earth Resource Utilization).
Author information
Authors and Affiliations
Contributions
YC and MT conceived and designed the research. MT and DZ performed the experiments. MT, XSC, LYL, HZW, and DZ conducted the data analysis. SSX provided cell lines and clinical samples. MT drafted the manuscript. YC, XLW, YZ, and SSX revised and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Tian, M., Chen, Xs., Li, Ly. et al. Inhibition of AXL enhances chemosensitivity of human ovarian cancer cells to cisplatin via decreasing glycolysis. Acta Pharmacol Sin 42, 1180–1189 (2021). https://doi.org/10.1038/s41401-020-00546-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-020-00546-8
Keywords
This article is cited by
-
Signaling networks and MiRNA crosstalk in ovarian cancer chemoresistance
Journal of Ovarian Research (2025)
-
AXL: shapers of tumor progression and immunosuppressive microenvironments
Molecular Cancer (2025)
-
AXL signaling in cancer: from molecular insights to targeted therapies
Signal Transduction and Targeted Therapy (2025)
-
Application of single cell sequencing technology in ovarian cancer research (review)
Functional & Integrative Genomics (2024)
-
AXL in cancer: a modulator of drug resistance and therapeutic target
Journal of Experimental & Clinical Cancer Research (2023)