Abstract
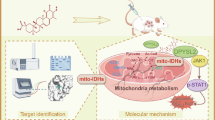
Celastrol is a triterpene derived from the traditional Chinese medicine Tripterygium wilfordii Hook f, which displays potential anticancer activity. In the present study, we investigated the anticancer effects of celastrol against clear cell renal cell carcinoma (ccRCC) and the underlying mechanisms. Using Cancer Genome Atlas (TCGA) database and genotype-tissue expression (GTEx) database we conducted a bioinformatics analysis, which showed that the mRNA levels of liver-X receptors α (LXRα) and ATP-binding cassette transporter A1 (ABCA1) in ccRCC tissues were significantly lower than those in adjacent normal tissues. This result was confirmed by immunoblotting analysis of 4 ccRCC clinical specimens, which showed that the protein expression of LXRα and ABCA1 was downregulated. Similar results were obtained in a panel of ccRCC cell lines (786-O, A498, SN12C, and OS-RC-2). In 786-O and SN12C cells, treatment with celastrol (0.25–2.0 μM) concentration-dependently inhibited the cell proliferation, migration, and invasion as well as the epithelial-mesenchymal transition (EMT) process. Furthermore, we demonstrated that celastrol inhibited the invasion of 786-O cells through reducing lipid accumulation; celastrol concentration-dependently promoted autophagy to reduce lipid storage. Moreover, we revealed that celastrol dramatically activated LXRα signaling, and degraded lipid droplets by inducing lipophagy in 786-O cells. Finally, celastrol promoted cholesterol efflux from 786-O cells via ABCA1. In high-fat diet-promoted ccRCC cell line 786-O xenograft model, administration of celastrol (0.25, 0.5, 1.0 mg·kg−1·d−1, for 4 weeks, i.p.) dose-dependently inhibited the tumor growth with upregulated LXRα and ABCA1 protein in tumor tissue. In conclusion, this study reveals that celastrol triggers lipophagy in ccRCC by activating LXRα, promotes ABCA1-mediated cholesterol efflux, suppresses EMT progress, and ultimately inhibits cell proliferation, migration, and invasion as well as tumor growth. Thus, our study provides evidence that celastrol can be used as a lipid metabolism-based anticancer therapeutic approach.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Change history
12 May 2025
The original online version of this article was revised: Fig. 2 has been updated.
14 May 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41401-025-01579-7
References
Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69:363–85.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30.
Choueiri TK. Clinical treatment decisions for advanced renal cell cancer. J Natl Compr Canc Netw. 2013;11:694–7.
Long J, Zhang CJ, Zhu N, Du K, Yin YF, Tan X, et al. Lipid metabolism and carcinogenesis, cancer development. Am J Cancer Res. 2018;8:778–91.
Wettersten HI, Aboud OA, Lara PN Jr, Weiss RH. Metabolic reprogramming in clear cell renal cell carcinoma. Nat Rev Nephrol. 2017;13:410–9.
Sanchez DJ, Simon MC. Genetic and metabolic hallmarks of clear cell renal cell carcinoma. Biochim Biophys Acta Rev Cancer. 2018;1870:23–31.
Cheng C, Geng F, Cheng X, Guo D. Lipid metabolism reprogramming and its potential targets in cancer. Cancer Commun. 2018;38:27.
Beloribi-Djefaflia S, Vasseur S, Guillaumond F. Lipid metabolic reprogramming in cancer cells. Oncogenesis. 2016;5:e189.
Qin W, Li C, Zheng W, Guo Q, Zhang Y, Kang M, et al. Inhibition of autophagy promotes metastasis and glycolysis by inducing ROS in gastric cancer cells. Oncotarget. 2015;6:39839–54.
Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, et al. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–5.
Villa GR, Hulce JJ, Zanca C, Bi J, Ikegami S, Cahill GL, et al. An LXR-cholesterol axis creates a metabolic co-dependency for brain cancers. Cancer Cell. 2016;30:683–93.
Carpenter KJ, Valfort AC, Steinauer N, Chatterjee A, Abuirqeba S, Majidi S, et al. LXR-inverse agonism stimulates immune-mediated tumor destruction by enhancing CD8 T-cell activity in triple negative breast cancer. Sci Rep. 2019;9:19530.
Tsui KH, Chung LC, Feng TH, Lee TY, Chang PL, Chen WT, et al. Divergent effect of liver X receptor agonists on prostate-specific antigen expression is dependent on androgen receptor in prostate carcinoma cells. Prostate. 2015;75:603–15.
Li T, Hu SM, Pang XY, Wang JF, Yin JY, Li FH, et al. The marine-derived furanone reduces intracellular lipid accumulation in vitro by targeting LXRα and PPARα. J Cell Mol Med. 2020;24:3384–98.
Tavazoie MF, Pollack I, Tanqueco R, Ostendorf BN, Reis BS, Gonsalves FC, et al. LXR/ApoE activation restricts innate immune suppression in cancer. Cell. 2018;172:825–40.
Liu J, Lee J, Salazar Hernandez MA, Mazitschek R, Ozcan U. Treatment of obesity with celastrol. Cell. 2015;161:999–1011.
Ouimet M, Franklin V, Mak E, Liao X, Tabas I, Marcel YL. Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metab. 2011;13:655–67.
Guo D, Zhang W, Yang H, Bi J, Xie Y, Cheng B, et al. Celastrol induces necroptosis and ameliorates inflammation via targeting biglycan in human gastric carcinoma. Int J Mol Sci. 2019;20:5716.
Hsieh MJ, Wang CW, Lin JT, Chuang YC, Hsi YT, Lo YS, et al. Celastrol, a plant-derived triterpene, induces cisplatin-resistance nasopharyngeal carcinoma cancer cell apoptosis though ERK1/2 and p38 MAPK signaling pathway. Phytomedicine. 2019;58:152805.
Chang W, He W, Li PP, Song SS, Yuan PF, Lu JT, et al. Protective effects of Celastrol on diethylnitrosamine-induced hepatocellular carcinoma in rats and its mechanisms. Eur J Pharmacol. 2016;784:173–80.
Feng X, Guan D, Auen T, Choi JW, Salazar Hernández MA, Lee J, et al. IL1R1 is required for celastrol’s leptin-sensitization and antiobesity effects. Nat Med. 2019;25:575–82.
Zhao M, Bu Y, Feng J, Zhang H, Chen Y, Yang G, et al. SPIN1 triggers abnormal lipid metabolism and enhances tumor growth in liver cancer. Cancer Lett. 2020;470:54–63.
Cao Q, Bai P. Role of autophagy in renal cancer. J Cancer. 2019;10:2501–9.
Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–75.
Santos CR, Schulze A. Lipid metabolism in cancer. FEBS J. 2012;279:2610–23.
Luo X, Cheng C, Tan Z, Li N, Tang M, Yang L, et al. Emerging roles of lipid metabolism in cancer metastasis. Mol Cancer. 2017;16:76. https://doi.org/10.1186/s12943-017-0646-3.
Neumann CKA, Silver DJ, Venkateshwari V, Zhang R, Traughber CA, Przybycin C, et al. MBOAT7-driven phosphatidylinositol remodeling promotes the progression of clear cell renal carcinoma. Mol Metab. 2020;34:136–45.
Wang K, Xu T, Ruan H, Xiao H, Liu J, Song Z, et al. LXRα promotes cell metastasis by regulating the NLRP3 inflammasome in renal cell carcinoma. Cell Death Dis. 2019;10:159.
Liang X, Cao Y, Xiang S, Xiang Z. LXRα-mediated downregulation of EGFR suppress colorectal cancer cell proliferation. J Cell Biochem. 2019;120:17391–404.
Smith B, Land H. Anticancer activity of the cholesterol exporter ABCA1 gene. Cell Rep. 2012;2:580–90.
Nieto MA, Huang RY, Jackson RA, Thiery JP. EMT: 2016. Cell. 2016;166:21–45.
Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017;168:670–91.
Serrano-Gomez SJ, Maziveyi M, Alahari SK. Regulation of epithelial-mesenchymal transition through epigenetic and post-translational modifications. Mol Cancer. 2016;15:18.
Choi SK, Park S, Jang S, Cho HH, Lee S, You S, et al. Cascade regulation of PPARγ(2) and C/EBPα signaling pathways by celastrol impairs adipocyte differentiation and stimulates lipolysis in 3T3-L1 adipocytes. Metabolism. 2016;65:646–54.
González-Chavarría I, Fernandez E, Gutierrez N, González-Horta EE, Sandoval F, Cifuentes P, et al. LOX-1 activation by oxLDL triggers an epithelial mesenchymal transition and promotes tumorigenic potential in prostate cancer cells. Cancer Lett. 2018;414:34–43.
Xu LN, Zhao N, Chen JY, Ye PP, Nan XW, Zhou HH, et al. Celastrol inhibits the growth of ovarian cancer cells in vitro and in vivo. Front Oncol. 2019;9:2. https://doi.org/10.3389/fonc.2019.00002.
Pang X, Yi Z, Zhang J, Lu B, Sung B, Qu W, et al. Celastrol suppresses angiogenesis-mediated tumor growth through inhibition of AKT/mammalian target of rapamycin pathway. Cancer Res. 2010;70:1951–9.
Liu X, Zhao P, Wang X, Wang L, Zhu Y, Song Y, et al. Celastrol mediates autophagy and apoptosis via the ROS/JNK and Akt/mTOR signaling pathways in glioma cells. J Exp Clin Cancer Res. 2019;38:184.
Tan YQ, Zhang J, Zhou G. Autophagy and its implication in human oral diseases. Autophagy. 2017;13:225–36.
Lizaso A, Tan KT, Lee YH. β-adrenergic receptor-stimulated lipolysis requires the RAB7-mediated autolysosomal lipid degradation. Autophagy. 2013;9:1228–43.
Martinez-Vicente M, Talloczy Z, Wong E, Tang G, Koga H, Kaushik S, et al. Cargo recognition failure is responsible for inefficient autophagy in Huntington’s disease. Nat Neurosci. 2010;13:567–76.
Texada MJ, Malita A, Christensen CF, Dall KB, Faergeman NJ, Nagy S, et al. Autophagy-mediated cholesterol trafficking controls steroid production. Dev Cell. 2019;48:659–71.
Shibata M, Yoshimura K, Furuya N, Koike M, Ueno T, Komatsu M, et al. The MAP1-LC3 conjugation system is involved in lipid droplet formation. Biochem Biophys Res Commun. 2009;382:419–23.
Shibata M, Yoshimura K, Tamura H, Ueno T, Nishimura T, Inoue T, et al. LC3, a microtubule-associated protein1A/B light chain3, is involved in cytoplasmic lipid droplet formation. Biochem Biophys Res Commun. 2010;393:274–9.
He P, Smith A, Gelissen IC, Ammit AJ. The effect of statins and the synthetic LXR agonist T0901317 on expression of ABCA1 transporter protein in human lung epithelial cell lines in vitro. Pharmacol Rep. 2019;71:1219–26.
Price NL, Rotllan N, Zhang X, Canfrán-Duque A, Nottoli T, Suarez Y, et al. Specific disruption of Abca1 targeting largely mimics the effects of miR-33 knockout on macrophage cholesterol efflux and atherosclerotic plaque development. Circ Res. 2019;124:874–80.
Frambach S, de Haas R, Smeitink JAM, Rongen GA, Russel FGM, Schirris TJJ. Brothers in arms: ABCA1- and ABCG1-mediated cholesterol efflux as promising targets in cardiovascular disease treatment. Pharmacol Rev. 2020;72:152–90.
Hsieh J, Koseki M, Molusky MM, Yakushiji E, Ichi I, Westerterp M, et al. TTC39B deficiency stabilizes LXR reducing both atherosclerosis and steatohepatitis. Nature. 2016;535:303–7.
Liu Y, Tang C. Regulation of ABCA1 functions by signaling pathways. Biochim Biophys Acta. 2012;1821:522–9.
Qian H, Zhao X, Cao P, Lei J, Yan N, Gong X. Structure of the human lipid exporter ABCA1. Cell. 2017;169:1228–39.
Zhang CJ, Zhu N, Liu Z, Shi Z, Long J, Zu XY, et al. Wnt5a/Ror2 pathway contributes to the regulation of cholesterol homeostasis and inflammatory response in atherosclerosis. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865:158547.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81973668, No. 81774130 and No. 81270359); the Natural Science Foundation of Hunan Province for Distinguished Young Scholars (No. 2018JJ1018); and the First-Class Discipline of Pharmaceutical Science of Hunan.
Author information
Authors and Affiliations
Contributions
LQ designed the research; CJZ and JL performed the research and analyzed the data; CJZ and YXW performed the in vivo experiments; NZ and HTW provided clinical samples; CJZ wrote the paper; and BYL and DFL revised the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
The original online version of this article was revised: Fig. 2 has been updated.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Cj., Zhu, N., Long, J. et al. Celastrol induces lipophagy via the LXRα/ABCA1 pathway in clear cell renal cell carcinoma. Acta Pharmacol Sin 42, 1472–1485 (2021). https://doi.org/10.1038/s41401-020-00572-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-020-00572-6
Keywords
This article is cited by
-
Targeting lipid metabolism: novel insights and therapeutic advances in pancreatic cancer treatment
Lipids in Health and Disease (2025)
-
Crosstalk between noncoding RNAs and autophagy in renal cell carcinoma: Deciphering molecular pathways and therapeutic prospects
Cancer Cell International (2025)
-
CircABCA1 promotes ccRCC by reprogramming cholesterol metabolism and facilitating M2 macrophage polarization through IGF2BP3-mediated stabilization of SCARB1 mRNA
Molecular Cancer (2025)
-
CARMN loss promotes VSMC-derived foam cell formation and atherosclerosis through transcriptional downregulation of autophagy
Cell Death & Disease (2025)
-
Targeting cholesterol metabolism: a promising therapy strategy for cancer
Acta Pharmacologica Sinica (2025)