Abstract
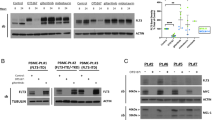
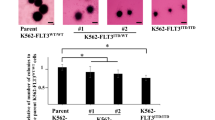
The kinase FLT3 internal tandem duplication (FLT3-ITD) is related to poor clinical outcomes of acute myeloid leukemia (AML). FLT3 inhibitors have provided novel strategies for the treatment of FLT3-ITD-positive AML. But they are limited by rapid development of acquired resistance and refractory in monotherapy. Recent evidence shows that inducing the degradation of FLT3-mutated protein is an attractive strategy for the treatment of FLT3-ITD-positive AML, especially those with FLT3 inhibitor resistance. In this study we identified Wu-5 as a novel USP10 inhibitor inducing the degradation of FLT3-mutated protein. We showed that Wu-5 selectively inhibited the viability of FLT3 inhibitor-sensitive (MV4-11, Molm13) and -resistant (MV4-11R) FLT3-ITD-positive AML cells with IC50 of 3.794, 5.056, and 8.386 μM, respectively. Wu-5 (1−10 μM) dose-dependently induced apoptosis of MV4-11, Molm13, and MV4-11R cells through the proteasome-mediated degradation of FLT3-ITD. We further demonstrated that Wu-5 directly interacted with and inactivated USP10, the deubiquitinase for FLT3-ITD in vitro (IC50 value = 8.3 µM) and in FLT3-ITD-positive AML cells. Overexpression of USP10 abrogated Wu-5-induced FLT3-ITD degradation and cell death. Also, the combined treatment of Wu-5 and crenolanib produced synergistic cell death in FLT3-ITD-positive cells via the reduction of both FLT3 and AMPKα proteins. In support of this, AMPKα inhibitor compound C synergistically enhanced the anti-leukemia effect of crenolanib, while AMPKα activator metformin inhibited the anti-leukemia effect of crenolanib. In summary, we demonstrate that Wu-5, a novel USP10 inhibitor, can overcome FLT3 inhibitor resistance and synergistically enhance the anti-AML effect of crenolanib through targeting FLT3 and AMPKα pathway.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Williams KM, Moore AR, Lucas PJ, Wang J, Bare CV, Gress RE. FLT3 ligand regulates thymic precursor cells and hematopoietic stem cells through interactions with CXCR4 and the marrow niche. Exp Hematol. 2017;52:40–49.
Brasel K, De Smedt T, Smith JL, Maliszewski CR. Generation of murine dendritic cells from flt3-ligand-supplemented bone marrow cultures. Blood. 2000;96:3029–39.
Lagunas-Rangel FA, Chavez-Valencia V. FLT3-ITD and its current role in acute myeloid leukaemia. Med Oncol. 2017;34:114.
Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374:2209–21.
Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–47.
Larrue C, Saland E, Boutzen H, Vergez F, David M, Joffre C, et al. Proteasome inhibitors induce FLT3-ITD degradation through autophagy in AML cells. Blood. 2016;127:882–92.
Bruner JK, Ma HS, Li L, Qin ACR, Rudek MA, Jones RJ, et al. Adaptation to TKI treatment reactivates ERK signaling in tyrosine kinase-driven leukemias and other malignancies. Cancer Res. 2017;77:5554–63.
Ju HQ, Zhan G, Huang A, Sun Y, Wen S, Yang J, et al. ITD mutation in FLT3 tyrosine kinase promotes Warburg effect and renders therapeutic sensitivity to glycolytic inhibition. Leukemia. 2017;31:2143–50.
Fan YC, Cao YN, Bai XS, Zhuang WF. The clinical significance of FLT3 ITD mutation on the prognosis of adult acute promyelocytic leukemia. Hematology. 2018;23:379–84.
Metzeler KH, Herold T, Rothenberg-Thurley M, Amler S, Sauerland MC, Gorlich D, et al. Spectrum and prognostic relevance of driver gene mutations in acute myeloid leukemia. Blood. 2016;128:686–98.
Weis TM, Marini BL, Bixby DL, Perissinotti AJ. Clinical considerations for the use of FLT3 inhibitors in acute myeloid leukemia. Crit Rev Oncol Hematol. 2019;141:125–38.
Shimada A. Hematological malignancies and molecular targeting therapy. Eur J Pharmacol. 2019;862:172641.
Skayneh H, Jishi B, Hleihel R, Hamieh M, Darwiche N, Bazarbachi A, et al. A critical review of animal models used in acute myeloid leukemia pathophysiology. Genes. 2019;10:614.
Jetani H, Garcia-Cadenas I, Nerreter T, Thomas S, Rydzek J, Meijide JB, et al. CAR T-cells targeting FLT3 have potent activity against FLT3−ITD+AML and act synergistically with the FLT3-inhibitor crenolanib. Leukemia. 2018;32:1168–79.
Zarrinkar PP, Gunawardane RN, Cramer MD, Gardner MF, Brigham D, Belli B, et al. AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML). Blood. 2009;114:2984–92.
Oshikawa G, Nagao T, Wu N, Kurosu T, Miura O. c-Cbl and Cbl-b Ligases Mediate 17-Allylaminodemethoxygeldanamycin-induced degradation of autophosphorylated Flt3 kinase with internal tandem duplication through the ubiquitin proteasome pathway. J Biol Chem. 2011;286:30263–73.
Makishima H, Sugimoto Y, Szpurka H, Clemente MJ, Ng KP, Muramatsu H, et al. CBL mutation-related patterns of phosphorylation and sensitivity to tyrosine kinase inhibitors. Leukemia. 2012;26:1547–54.
Buchwald M, Pietschmann K, Muller JP, Bohmer FD, Heinzel T, Kramer OH. Ubiquitin conjugase UBCH8 targets active FMS-like tyrosine kinase 3 for proteasomal degradation. Leukemia. 2010;24:1412–21.
Taylor SJ, Thien CBF, Dagger SA, Duyvestyn JM, Grove CS, Lee BH, et al. Loss of c-Cbl E3 ubiquitin ligase activity enhances the development of myeloid leukemia in FLT3-ITD mutant mice. Exp Hematol. 2015;43:191–206.
Weisberg EL, Schauer NJ, Yang J, Lamberto I, Doherty L, Bhatt S, et al. Inhibition of USP10 induces degradation of oncogenic FLT3. Nat Chem Biol. 2017;13:1207–15.
Akiyama H, Umezawa Y, Ishida S, Okada K, Nogami A, Miura O. Inhibition of USP9X induces apoptosis in FLT3-ITD-positive AML cells cooperatively by inhibiting the mutant kinase through aggresomal translocation and inducing oxidative stress. Cancer Lett. 2019;453:84–94.
Wu ZX, Zhuang HF, Yu QF, Zhang XZ, Jiang XD, Lu XY, et al. Homoharringtonine combined with the heat shock protein 90 inhibitor IPI504 in the treatment of FLT3-ITD acute myeloid leukemia. Transl Oncol. 2019;12:801–9.
Burslem GM, Song JY, Chen X, Hines J, Crews CM. Enhancing antiproliferative activity and selectivity of a FLT-3 inhibitor by proteolysis targeting chimera conversion. J Am Chem Soc. 2018;140:16428–32.
Xu X, Huang L, Zhang Z, Tong J, Mi J, Wu Y, et al. Targeting non-oncogene ROS pathway by alantolactone in B cell acute lymphoblastic leukemia cells. Life Sci. 2019;227:153–65.
Jing B, Liu M, Yang L, Cai HY, Chen JB, Li ZX, et al. Characterization of naturally occurring pentacyclic triterpenes as novel inhibitors of deubiquitinating protease USP7 with anticancer activity in vitro. Acta Pharmacol Sin. 2018;39:492–8.
Almqvist H, Axelsson H, Jafari R. CETSA screening identifies known and novel thymidylate synthase inhibitors and slow intracellular activation of 5-fluorouracil. Nat Commun. 2016;7:11040. https://doi.org/10.1038/ncomms11040.
Martinez Molina D, Nordlund P. The cellular thermal shift assay: a novel biophysical assay for in situ drug target engagement and mechanistic biomarker studies. Annu Rev Pharmacol Toxicol. 2016;56:141–61.
Pei S, Minhajuddin M, Adane B, Khan N, Stevens BM, Mack SC, et al. AMPK/FIS1-mediated mitophagy is required for self-renewal of human AML stem cells. Cell Stem Cell. 2018;23:86–100.e6.
Smith CC. The growing landscape of FLT3 inhibition in AML. Hematol Am Soc Hematol Educ Program. 2019;2019:539–47.
Elyamany G, Awad M, Alsuhaibani O, Fadalla K, Al Sharif O, Al Shahrani M, et al. FLT3 internal tandem duplication and D835 mutations in patients with acute lymphoblastic leukemia and its clinical significance. Mediterr J Hematol Infect Dis. 2014;6:e2014038.
Daver N, Schlenk RF, Russell NH, Levis MJ. Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia. 2019;33:299–312.
Feng JH, Fang H, Wang XJ, Jia YP, Zhang L, Jiao J, et al. Discovery of N-hydroxy-4-(3-phenylpropanamido) benzamide derivative 5j, a novel histone deacetylase inhibitor, as a potential therapeutic agent for human breast cancer. Cancer Biol Ther. 2011;11:477–89.
Molloy ME, White BEP, Gherezghiher T, Michalsen BT, Xiong R, Patel H, et al. Novel selective estrogen mimics for the treatment of tamoxifen-resistant breast cancer. Mol Cancer Ther. 2014;13:2515–26.
Gliyazova NS, Ibeanu GC. The chemical molecule B355252 is neuroprotective in an in vitro model of Parkinson’s disease. Cell Mol Neurobiol. 2016;36:1109–22.
Liao YN, Guo ZQ, Xia XH, Liu Y, Huang CY, Jiang LL, et al. Inhibition of EGFR signaling with Spautin-1 represents a novel therapeutics for prostate cancer. J Exp Clin Cancer Res. 2019;38:157. https://doi.org/10.1186/s13046-019-1165-4.
Chen Q, Hang YY, Zhang TT, Tan L, Li SD, Jin YL. USP10 promotes proliferation and migration and inhibits apoptosis of endometrial stromal cells in endometriosis through activating the Raf-1/MEK/ERK pathwa. Am J Physiol-Cell Physiol. 2018;315:C863–C72.
Liu J, Xia H, Kim M, Xu L, Li Y, Zhang L, et al. Beclin1 controls the levels of p53 by regulating the deubiquitination activity of USP10 and USP13. Cell. 2011;147:223–34.
Xu WB, Wei W, Yu Q, Wu C, Ye CJ, Wu YL, et al. Arsenic trioxide and bortezomib interact synergistically to induce apoptosis in chronic myelogenous leukemia cells resistant to imatinib mesylate through Bcr/Abl-dependent mechanisms. Mol Med Rep. 2014;10:1519–24.
Deng M, Yang X, Qin B, Liu T, Zhang H, Guo W, et al. Deubiquitination and activation of AMPK by USP10. Mol Cell. 2016;61:614–24.
Acknowledgements
This work was supported in part by grants from the National Key Research and Development Program of China (No. 2017YFA0505202), the National Basic Research Program of China (973 Program) (No. 2015CB910403), the National Natural Science Foundation of China (Nos. 81570118, 81700475, 81570112, 81670139, and 21602133), the National Natural Science Foundation Youths of China (No. 81700157), Innovative research team of high-level local universities in Shanghai (SSMU-ZDCX20181202).
Author information
Authors and Affiliations
Contributions
YLW and WL conceived and designed the study. MY and ZXF performed the experiments, collected data, conducted preliminary analysis of data and wrote the initial article. WWW, YZ, ZLB, ML, XHX, ZLZ, XMZ, YC, YYW, HL, HZX and YZW assisted in data analysis, manuscript writing and editing. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Yu, M., Fang, Zx., Wang, Ww. et al. Wu-5, a novel USP10 inhibitor, enhances crenolanib-induced FLT3-ITD-positive AML cell death via inhibiting FLT3 and AMPK pathways. Acta Pharmacol Sin 42, 604–612 (2021). https://doi.org/10.1038/s41401-020-0455-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-020-0455-x
Keywords
This article is cited by
-
USP10 promotes glioma stem cell maintenance and glioblastoma growth by antagonizing DTX3L-mediated SATB2 ubiquitination
Nature Communications (2026)
-
Decursin induces FLT3-ITD acute myeloid leukemia cell apoptosis by increasing the expression of the ubiquitin-conjugase UBE2L6
Cell Communication and Signaling (2025)
-
Deubiquitinating enzymes: potential regulators of the tumor microenvironment and implications for immune evasion
Cell Communication and Signaling (2024)
-
Deubiquitinase USP10 promotes osteosarcoma autophagy and progression through regulating GSK3β-ULK1 axis
Cell & Bioscience (2024)
-
Ubiquitin-specific protease 10 determines colorectal cancer outcome by modulating epidermal growth factor signaling via inositol polyphosphate-4-phosphatase type IIB
Oncogenesis (2024)