Abstract
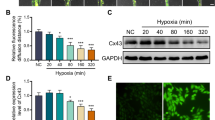
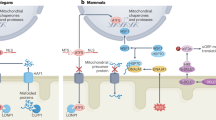
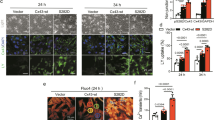
Cardiac hypertrophy (CH) is characterized by an increase in cardiomyocyte size, and is the most common cause of cardiac-related sudden death. A decrease in gap junction (GJ) coupling and mitochondrial dysfunction are important features of CH, but the mechanisms of decreased coupling and energy impairment are poorly understood. It has been reported that GJA1-20k has a strong tropism for mitochondria and is required for the trafficking of connexin 43 (Cx43) to cell–cell borders. In this study, we investigated the effects of GJA1-20k on Cx43 GJ coupling and mitochondrial function in the pathogenesis of CH. We performed hematoxylin–eosin (HE) and Masson staining, and observed significant CH in 18-week-old male spontaneously hypertensive rats (SHRs) compared to age-matched normotensive Wistar-Kyoto (WKY) rats. In cardiomyocytes from SHRs, the levels of Cx43 at the intercalated disc (ID) and the expression of GJA1-20k were significantly reduced, whereas JAK-STAT signaling was activated. Furthermore, the SHR rats displayed suppressed mitochondrial GJA1-20k and mitochondrial biogenesis. Administration of valsartan (10 mg· \({\rm{kg}}^{-1_{.}}\) d−1, i.g., for 8 weeks) prevented all of these changes. In neonatal rat cardiomyocytes (NRCMs), overexpression of GJA1-20k attenuated Ang II-induced cardiomyocyte hypertrophy and caused elevated levels of GJ coupling at the cell–cell borders. Pretreatment of NRCMs with the Jak2 inhibitor AG490 (10 µM) blocked Ang II-induced reduction in GJA1-20k expression and Cx43 gap junction formation; knockdown of Jak2 in NRCMs significantly lessened Ang II-induced cardiomyocyte hypertrophy and normalized GJA1-20k expression and Cx43 gap junction formation. Overexpression of GJA1-20k improved mitochondrial membrane potential and respiration and lowered ROS production in Ang II-induced cardiomyocyte hypertrophy. These results demonstrate the importance of GJA1-20k in regulating gap junction formation and mitochondrial function in Ang II-induced cardiomyocyte hypertrophy, thus providing a novel therapeutic strategy for patients with cardiomyocyte hypertrophy.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79.
Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600.
Lips DJ, dewindt LJ, Van Kraaij DJ, Doevendans PA. Molecular determinants of myocardial hypertrophy and failure: alternative pathways for beneficial and maladaptive hypertrophy. Eur Heart J. 2003;24:883–96.
Ritter O, Neyses L. The molecular basis of myocardial hypertrophy and heart failure. Trends Mol Med. 2003;9:313–21.
Chien KR, Knowlton KU. Regulation of cardiac gene expression during myocardial growth and hypertrophy: molecular studies of an adaptive physiologic response. FASEB J 1991;5:3037–46.
Bruzzone HarrisAL, Locke D. Connexins: a guide. Mol Biotechnol 2009;43:12300–9.
Saffitz JE, Hames KY, Kanno S. Remodeling of gap junctions in ischemic and nonischemic forms of heart disease. J Membr Biol. 2007;218:65–71.
Severs NJ, Bruce AF, Dupont E, Rothery S. Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc Res 2008;80:9–19.
Peters NS. New insights into myocardial arrhythmogenesis: distribution of gap-junctional coupling in normal, ischaemic and hypertrophied hearts. Clin Sci. 1996;90:447–52.
Kostin S, Rieger M, Dammer S, Hein S, Richter M, Klövekorn WP, et al. Gap junction remodeling and altered connexin43 expression in the failing human heart. Mol Cell Biochem. 2003;242:135–44.
Kitamura H, Ohnishi Y, Yoshida A, Okajima K, Azumi H, Ishida A, et al. Heterogeneous loss of connexin43 protein in nonischemic dilated cardiomyopathy with ventricular tachycardia. J Cardiovasc Electrophysiol. 2002;13:865–70.
Dupont E, Matsushita T, Kaba RA, Vozzi C, Coppen SR, Khan N, et al. Altered connexin expression in human congestive heart failure. J Mol Cell Cardiol. 2001;33:359–71.
Angeliki A, Sudhir K, Eva P, Anne KA, Edward A, Zhenzhen L, et al. Identification of a new modulator of the intercalated disc in a zebrafish model of arrhythmogenic cardiomyopathy. Sci Transl Med. 2014;6:240–74.
Remo BF, Giovannone S, Fishman GI. Connexin43 cardiac gap junction remodeling: lessons from genetically engineered murine models. J Membr Biol. 2012;245:275–81.
Spach MS, Boineau JP. Microfibrosis produces electrical load variations due to loss of side-to-side cell connections: a major mechanism of structural heart disease arrhythmias. Pacing Clin Electrophysiol. 1997;20:397–413.
Zhou LY, Liu JP, Wang K, Gao J, Ding SL, Jiao JQ, et al. Mitochondrial function in cardiac hypertrophy. Int J Cardiol. 2013;167:1118–25.
Wikman-Coffelt J, Parmley WW, Mason DT. The cardiac hypertrophy process. Analyses of factors determining pathological vs. physiological development. Circ Res. 1979;45:697–707.
Vinken M, Decrock E, Leybaert L, Bultynck G, Himpens B, Vanhaecke T, et al. Non-channel functions of connexins in cell growth and cell death. Biochim Biophys Acta. 2012;1818:2002–8.
Kardami E, Dang X, Iacobas DA, Nickel BE, Jeyaraman M, Srisakuldee W, et al. The role of connexins in controlling cell growth and gene expression. Prog Biophys Mol Biol. 2007;94:245–64.
Jiang JX, Penuela S. Connexin and pannexin channels in cancer. BMC Mol Cell Biol. 2016;17:12–26.
Aasen T, Mesnil M, Naus CC, Lampe PD, Laird DW. Gap junctions and cancer: communicating for 50 years. Nat Rev Cancer. 2016;16:775–88.
Smyth JW, Shaw RM. Autoregulation of connexin43 gap junction formation by internally translated isoforms. Cell Rep. 2013;5:611–8.
Fu Y, Zhang SS, Xiao S, Basheer A, Baum R, Epifantseva I, et al. Cx43 isoform GJA11-20k promotes microtubule dependent mitochondrial transport. Front Physiol 2017;8:905–17.
Basheer WA, Fu Y, Shimura D, Xiao S, Agvanian S, Hernandez DM, et al. Stress response protein GJA1-20k promotes mitochondrial biogenesis, metabolic quiescence, and cardioprotection against ischemia/reperfusion injury. JCI Insight 2018;3:378–90.
Yang D, Xi J, Xing Y, Tang X, Dai X, Li K, et al. A new method for neonatal rat ventricular myocyte purification using superparamagnetic iron oxide particles. Int J Cardiol. 2018;270:293–301.
Wang Y, Wang F, Yang D. Berberine in combination with yohimbine attenuates sepsis-induced neutrophil tissue infiltration and multiorgan dysfunction partly via IL-10-mediated inhibition of CCR2 expression in neutrophils. Int Immunopharmacol. 2016;35:217–25.
Xiang Y, Wang Q, Guo Y, Ge H, Fu Y, Wang X, et al. Cx32 exerts anti-apoptotic and pro-tumor effects via the epidermal growth factor receptor pathway in hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38:145–60.
Doser TA, Turdi S, Thomas DP, Epstein PN, Li SY, Ren J. Transgenic overexpression of aldehyde dehydrogenase-2 rescues chronic alcohol intake-induced myocardial hypertrophy and contractile dysfunction. Circulation 2009;119:1941–9.
Shi Z, Wei Z, Li J, Yuan S, Pan B, Cao F, et al. Identification and verification of candidate genes regulating neural stem cells behavior under hypoxia. Cell Physiol Biochem. 2018;47:212–22.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–50.
Shah BH, Catt KJ. A central role of EGF receptor transactivation in angiotensin II-induced cardiac hypertrophy. Trends Pharmacol Sci. 2003;24:239–44.
Salat-Canela C, Sesé M, Peula C, Cajal SRY, Aasen T. Internal translation of the connexin 43 transcript. Cell Commun Signal. 2014;12:31–44.
Chatterjee B, Chin AJ, Valdimarsson G, Finis C, Sonntag JM, Choi BY, et al. Developmental regulation and expression of the zebrafish connexin43 gene. Dev Dynam. 2005;233:890–906.
Basheer WA, Xiao S, Epifantseva I, Fu Y, Kleber AG, Hong T, et al. GIA1-20k arranges actin to guide Cx43 delivery to cardiac intercalated discs. Circ Res 2017;121:1069–80.
James CC, Zeitz MJ, Calhoun PJ, Lamouille S, Smyth JW. Altered translation initiation of GJA1 limits gap junction formation during epithelial–mesenchymal transition. Mol Biol Cell. 2018;29:797–808.
Booz GW, Day JN, Baker KM. Interplay between the cardiac renin angiotensin system and JAK-STAT signaling: role in cardiac hypertrophy, ischemia/reperfusion dysfunction, and heart failure. J Mol Cell Cardiol. 2002;34:1443–53.
Mascareno E, Dhar M, Siddiqui MA. Signal transduction and activator of transcription (STAT) protein-dependent activation of angiotensinogen promoter: a cellular signal for hypertrophy in cardiac muscle. Proc Natl Acad Sci USA. 1998;95:5590–4.
Mascareno E, Siddiqui MA. The role of Jak/STAT signaling in heart tissue renin–angiotensin system. Mol Cell Biochem. 2000;212:171–5.
Rohini A, Agrawal N, Koyani CN, Singh R. Molecular targets and regulators of cardiac hypertrophy. Pharmacol Res. 2010;61:269–80.
Kunisada K, Negoro S, Tone E, Funamoto M, Osugi T, Yamada S, et al. Signal transducer and activator of transcription 3 in the heart transduces not only a hypertrophic signal but a protective signal against doxorubicin-induced cardiomyopathy. Proc Natl Acad Sci USA. 2000;97:315–9.
Harada M, Qin Y, Takano H, Minamino T, Zou Y, Toko H, et al. G-CSF prevents cardiac remodeling after myocardial infarction by activating the Jak-Stat pathway in cardiomyocytes. Nat Med 2005;11:305–11.
Zeitz MJ, Calhoun PJ, James CC, Taetzsch T, George KK, Robel S, et al. Dynamic UTR usage regulates alternative translation to modulate gap junction formation during stress and aging. Cell Rep. 2019;27:2737–47.
Cutler MJ, Jeyaraj D, Rosenbaum DS. Cardiac electrical remodeling in health and disease. Trends Pharmacol Sci. 2011;32:174–80.
Pozlzing S, Rosenbaum DS. Altered Connexin43 expression produces arrhythmia substrate in heart failure. Am J Physiol Heart Circ Physiol. 2004;287:1762–70.
Akar FG, Nass RD, Hahn S, Tomaselli GF. Dynamic changes in conduction velocity and gap junction properties during development of pacing-induced heart failure. Am J Physiol Heart Circ Physiol. 2007;293:1223–30.
Akar FG, Spragg DD, Tunin RS, Kass DA, Tomaselli GF. Mechanisms underlying conduction slowing and arrhythmogenesis in nonischemic dilated cardiomyopathy. Circ Res. 2004;95:717–25.
Danik SB, Liu F, Zhang J. Modulation of cardiac gap junction expression and arrhythmic susceptibility. Circ Res. 2004;95:1035–41.
Gutstein DE, Morley GE, Tamaddon H, Vaidya D, Schneider MD, Chen J, et al. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43. Circ Res. 2001;88:333–9.
Gutstein DE, Morley GE, Vaidya D, Fishman GI. Heterogeneous expression of gap junction channels in the heart leads to conduction defects and ventricular dysfunction. Circulation. 2001;104:1194–9.
Leybaert L, Lampe PD, Dhein S, Kwak BR, Ferdinandy P, Beyer EC, et al. Connexins in cardiovascular and neurovascular health and disease: pharmacological implications. Pharmacol Rev. 2017;69:396–478.
Hesketh GG, Shah MH, Halperin VL, Cooke CA, Akar FG, et al. Ultrastructure and regulation of lateralized connexin43 in the failing heart. Circ Res. 2010;106:1153–63.
Dhein S, Manicone N, Müller A, Gerwin R, Ziskoven U, Irankhahi A, et al. A new synthetic antiarrhythmic peptide reduces dispersion of epicardial activation recovery interval and diminishes alterations of epicardial activation patterns induced by regional ischemia. Naunyn Schmiedebergs Arch Pharmacol. 1994;350:174–84.
Lin X, Zemlin C, Hennan JK, Petersen JS, Veenstra RD. Enhancement of ventricular gap-junction coupling by rotigaptide. Cardiovasc Res. 2008;79:416–26.
Clarke TC, Thomas D, Petersen JS, Evans WH, Martin PE. The antiarrhythmic peptide rotigaptide (ZP123) increases gap junction intercellular communication in cardiac myocytes and HeLa cells expressing connexin43. Br J Pharmacol. 2006;147:486–95.
Jorgensen NR, Teilmann SC, Henriksen Z, Meier E, Hansen SS, Jensen JE, et al. The antiarrhythmic peptide analog rotigaptide (ZP123) stimulates gap junction intercellular communication in human osteoblasts and prevents decrease in femoral trabecular bone strength in ovariectomized rats. Endocrinology. 2005;146:4745–54.
Rossman EI, Liu K, Morgan GA, Swillo RE, Krueger JA, Gardell SJ, et al. The gap junction modifier, improves conduction and reduces atrial fibrillation/flutter in the canine sterile pericarditis model. J Pharmacol Exp Ther. 2009;329:1127–33.
Laurent G, Leong-Poi H, Mangat I, Moe GW, Hu X, So PP, et al. Effects of chronic gap junction conduction-enhancing antiarrhythmic peptide GAP-134 administration on experimental atrial fibrillation in dogs. Circ Arrhythmia Electrophysiol. 2009;2:171–8.
Boengler K, Bulic M, Schreckenberg R, Schlüter KD, Schulz R. The gap junction modifier ZP1609 decreases cardiomyocyte hypercontracture following ischaemia/reperfusion independent from mitochondrial connexin43. Br J Pharmacol. 2017;174:2060–73.
Skyschally A, Walter B, Schultz Hansen R, Heusch G. The antiarrhythmic dipeptide ZP1609 (danegaptide) when given at reperfusion reduces myocardial infarct size in pigs. Naunyn Schmiedebergs Arch Pharmacol. 2013;386:383–91.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant No. 81473234), the Joint Fund of the National Natural Science Foundation of China (grant No. U1303221), the Guangdong Basic and Applied Basic Research Foundation (grant No. 2019A1515012215), and a grant from the Department of Science and Technology of Guangdong Province (grant No. 20160908).
Author information
Authors and Affiliations
Contributions
LT and QW conceived and designed the study. YLF and FHP performed the siRNA and cDNA transfection experiments, qPCR and Western blot analyses. NZZ and QL performed the flow cytometry experiments and immunofluorescence and collected and analyzed the data. SYC was involved in cell culture and made substantial contributions to the acquisition and interpretation of the data. YLF wrote the manuscript. LT and QW revised and amended the draft. QW contributed to the final approval of the version to be published. All authors read and approved the final manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy and integrity of all parts of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Fu, Yl., Tao, L., Peng, Fh. et al. GJA1-20k attenuates Ang II-induced pathological cardiac hypertrophy by regulating gap junction formation and mitochondrial function. Acta Pharmacol Sin 42, 536–549 (2021). https://doi.org/10.1038/s41401-020-0459-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-020-0459-6
Keywords
This article is cited by
-
Connexin 43 regulates intercellular mitochondrial transfer from human mesenchymal stromal cells to chondrocytes
Stem Cell Research & Therapy (2024)
-
Mitochondria and metabolic transitions in cardiomyocytes: lessons from development for stem cell-derived cardiomyocytes
Stem Cell Research & Therapy (2021)
-
Pharmacological characterization of MT-1207, a novel multitarget antihypertensive agent
Acta Pharmacologica Sinica (2021)
-
Allisartan isoproxil reduces mortality of stroke-prone rats and protects against cerebrovascular, cardiac, and aortic damage
Acta Pharmacologica Sinica (2021)