Abstract
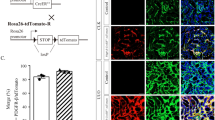
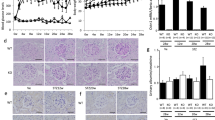
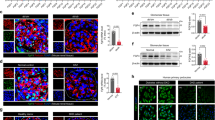
We previously found that polydatin could attenuate renal oxidative stress in diabetic mice and improve renal fibrosis. Recent evidence shows that NADPH oxidase 4 (Nox4)-derived reactive oxygen species (ROS) contribute to inflammatory and fibrotic processes in diabetic kidneys. In this study we investigated whether polydatin attenuated renal fibrosis by regulating Nox4 in vitro and in vivo. In high glucose-treated rat glomerular mesangial cells, polydatin significantly decreased the protein levels of Nox4 by promoting its K48-linked polyubiquitination, thus inhibited the production of ROS, and eventually decreasing the expression of fibronectin (FN) and intercellular adhesion molecule-1 (ICAM-1), the main factors that exacerbate diabetic renal fibrosis. Overexpression of Nox4 abolished the inhibitory effects of polydatin on FN and ICAM-1 expression. In addition, the expression of Connexin32 (Cx32) was significantly decreased, which was restored by polydatin treatment. Cx32 interacted with Nox4 and reduced its protein levels. Knockdown of Cx32 abolished the inhibitory effects of polydatin on the expression of FN and ICAM-1. In the kidneys of streptozocin-induced diabetic mice, administration of polydatin (100 mg·kg−1·d−1, ig, 6 days a week for 12 weeks) increased Cx32 expression and reduced Nox4 expression, decreased renal oxidative stress levels and the expression of fibrotic factors, eventually attenuating renal injury and fibrosis. In conclusion, polydatin promotes K48-linked polyubiquitination and degradation of Nox4 by restoring Cx32 expression, thereby decreasing renal oxidative stress levels and ultimately ameliorating the pathological progress of diabetic renal fibrosis. Thus, polydatin reduces renal oxidative stress levels and attenuates diabetic renal fibrosis through regulating the Cx32-Nox4 signaling pathway.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Kanwar YS, Sun L, Xie P, Liu FY, Chen S. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu Rev Pathol. 2011;6:395–423.
Kim Y, Park CW. New therapeutic agents in diabetic nephropathy. Korean J Intern Med. 2017;32:11–25.
Alsaad KO, Herzenberg AM. Distinguishing diabetic nephropathy from other causes of glomerulosclerosis: an update. J Clin Pathol. 2007;60:18–26.
Jha JC, Banal C, Chow BS, Cooper ME, Jandeleit-Dahm K. Diabetes and kidney disease: role of oxidative stress. Antioxid Redox Signal. 2016;25:657–84.
Yang Q, Wu FR, Wang JN, Gao L, Jiang L, Li HD, et al. Nox4 in renal diseases: an update. Free Radic Biol Med. 2018;124:466–72.
Gorin Y, Block K. Nox4 and diabetic nephropathy: with a friend like this, who needs enemies? Free Radic Biol Med. 2013;61:130–42.
Jha JC, Gray SP, Barit D, Okabe J, El-Osta A, Namikoshi T, et al. Genetic targeting or pharmacologic inhibition of NADPH oxidase nox4 provides renoprotection in long-term diabetic nephropathy. J Am Soc Nephrol. 2014;25:1237–54.
Rhee EP. NADPH oxidase 4 at the nexus of diabetes, reactive oxygen species, and renal metabolism. J Am Soc Nephrol. 2016;27:337–9.
Palumbo S, Shin YJ, Ahmad K, Desai AA, Quijada H, Mohamed M, et al. Dysregulated Nox4 ubiquitination contributes to redox imbalance and age-related severity of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2017;312:L297–308.
Tsubouchi K, Araya J, Minagawa S, Hara H, Ichikawa A, Saito N, et al. Azithromycin attenuates myofibroblast differentiation and lung fibrosis development through proteasomal degradation of NOX4. Autophagy. 2017;13:1420–34.
Oyamada M, Takebe K, Oyamada Y. Regulation of connexin expression by transcription factors and epigenetic mechanisms. Biochim Biophys Acta. 2013;1828:118–33.
King TJ, Lampe PD. Mice deficient for the gap junction protein Connexin32 exhibit increased radiation-induced tumorigenesis associated with elevated mitogen-activated protein kinase (p44/Erk1, p42/Erk2) activation. Carcinogenesis. 2004;25:669–80.
Cogliati B, Crespo Yanguas S, Da Silva TC, Aloia TP, Nogueira MS, Real-Lima MA, et al. Connexin32 deficiency exacerbates carbon tetrachloride-induced hepatocellular injury and liver fibrosis in mice. Toxicol Mech Methods. 2016;26:362–70.
Tiburcio TC, Willebrords J, da Silva TC, Pereira IV, Nogueira MS, Crespo Yanguas S, et al. Connexin32 deficiency is associated with liver injury, inflammation and oxidative stress in experimental non-alcoholic steatohepatitis. Clin Exp Pharmacol Physiol. 2017;44:197–206.
Pitre DA, Seifert JL, Bauer JA. Perineurium inflammation and altered connexin isoform expression in a rat model of diabetes related peripheral neuropathy. Neurosci Lett. 2001;303:67–71.
Arora MK, Singh UK. Molecular mechanisms in the pathogenesis of diabetic nephropathy: an update. Vasc Pharmacol. 2013;58:259–71.
Haefliger JA, Krattinger N, Martin D, Pedrazzini T, Capponi A, Doring B, et al. Connexin43-dependent mechanism modulates renin secretion and hypertension. J Clin Invest. 2006;116:405–13.
Chen Z, Sun X, Chen Q, Lan T, Huang K, Xiao H, et al. Connexin32 ameliorates renal fibrosis in diabetic mice by promoting K48-linked NADPH oxidase 4 polyubiquitination and degradation. Br J Pharmacol. 2020;177:145–60.
Ravagnan G, De Filippis A, Carteni M, De Maria S, Cozza V, Petrazzuolo M, et al. Polydatin, a natural precursor of resveratrol, induces beta-defensin production and reduces inflammatory response. Inflammation. 2013;36:26–34.
Du QH, Peng C, Zhang H. Polydatin: a review of pharmacology and pharmacokinetics. Pharm Biol. 2013;51:1347–54.
Hao J, Chen C, Huang K, Huang J, Li J, Liu P, et al. Polydatin improves glucose and lipid metabolism in experimental diabetes through activating the Akt signaling pathway. Eur J Pharmacol. 2014;745:152–65.
Gong W, Li J, Chen Z, Huang J, Chen Q, Cai W, et al. Polydatin promotes Nrf2-ARE anti-oxidative pathway through activating CKIP-1 to resist HG-induced up-regulation of FN and ICAM-1 in GMCs and diabetic mice kidneys. Free Radic Biol Med. 2017;106:393–405.
Huang K, Chen C, Hao J, Huang J, Wang S, Liu P, et al. Polydatin promotes Nrf2-ARE anti-oxidative pathway through activating Sirt1 to resist AGEs-induced upregulation of fibronetin and transforming growth factor-beta1 in rat glomerular messangial cells. Mol Cell Endocrinol. 2015;399:178–89.
Chen Z, Xie X, Huang J, Gong W, Zhu X, Chen Q, et al. Connexin43 regulates high glucose-induced expression of fibronectin, ICAM-1 and TGF-beta1 via Nrf2/ARE pathway in glomerular mesangial cells. Free Radic Biol Med. 2017;102:77–86.
Jiang Q, Liu P, Wu X, Liu W, Shen X, Lan T, et al. Berberine attenuates lipopolysaccharide-induced extracelluar matrix accumulation and inflammation in rat mesangial cells: involvement of NF-kappaB signaling pathway. Mol Cell Endocrinol. 2011;331:34–40.
Abboud HE. Mesangial cell biology. Exp Cell Res. 2012;318:979–85.
Schlondorff D, Banas B. The mesangial cell revisited: no cell is an island. J Am Soc Nephrol. 2009;20:1179–87.
Cove-Smith A, Hendry BM. The regulation of mesangial cell proliferation. Nephron Exp Nephrol. 2008;108:e74–9.
Lai Y, Zhou C, Huang P, Dong Z, Mo C, Xie L, et al. Polydatin alleviated alcoholic liver injury in zebrafish larvae through ameliorating lipid metabolism and oxidative stress. J Pharmacol Sci. 2018;138:46–53.
Lv T, Shen L, Yang L, Diao W, Yang Z, Zhang Y, et al. Polydatin ameliorates dextran sulfate sodium-induced colitis by decreasing oxidative stress and apoptosis partially via Sonic hedgehog signaling pathway. Int Immunopharmacol. 2018;64:256–63.
Lv R, Du L, Zhang L, Zhang Z. Polydatin attenuates spinal cord injury in rats by inhibiting oxidative stress and microglia apoptosis via Nrf2/HO-1 pathway. Life Sci. 2019;217:119–27.
Jha JC, Banal C, Okabe J, Gray SP, Hettige T, Chow BSM, et al. NADPH oxidase Nox5 accelerates renal injury in diabetic nephropathy. Diabetes. 2017;66:2691–703.
Ni Z, Tao L, Xiaohui X, Zelin Z, Jiangang L, Zhao S, et al. Polydatin impairs mitochondria fitness and ameliorates podocyte injury by suppressing Drp1 expression. J Cell Physiol. 2017;232:2776–87.
Sedeek M, Callera G, Montezano A, Gutsol A, Heitz F, Szyndralewiez C, et al. Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. Am J Physiol Renal Physiol. 2010;299:F1348–58.
Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82.
Amin MM, Rafiei N, Poursafa P, Ebrahimpour K, Mozafarian N, Shoshtari-Yeganeh B, et al. Association of benzene exposure with insulin resistance, SOD, and MDA as markers of oxidative stress in children and adolescents. Environ Sci Pollut Res Int. 2018;25:34046–52.
Hajiluian G, Abbasalizad Farhangi M, Nameni G, Shahabi P, Megari-Abbasi M. Oxidative stress-induced cognitive impairment in obesity can be reversed by vitamin D administration in rats. Nutr Neurosci. 2018;21:744–52.
Wang H, Gao N, Li Z, Yang Z, Zhang T. Autophagy alleviates melamine-induced cell death in PC12 cells via decreasing ROS level. Mol Neurobiol. 2016;53:1718–29.
New DD, Block K, Bhandhari B, Gorin Y, Abboud HE. IGF-I increases the expression of fibronectin by Nox4-dependent Akt phosphorylation in renal tubular epithelial cells. Am J Physiol Cell Physiol. 2012;302:C122–30.
Thallas-Bonke V, Jha JC, Gray SP, Barit D, Haller H, Schmidt HH, et al. Nox-4 deletion reduces oxidative stress and injury by PKC-alpha-associated mechanisms in diabetic nephropathy. Physiol Rep. 2014;2:e12192. https://doi.org/10.14814/phy2.12192.
Acknowledgements
This work was supported by research grants from the National Natural Science Foundation of China (grant number 81770816 and 81973375), the Key Project of Natural Science Foundation of Guangdong Province, China (grant number 2017A030311036), and the Youth Science Foundation of Guangxi Medical University, China (grant number GXMUYSF201903).
Author information
Authors and Affiliations
Contributions
ZQC conceived and designed the study, performed the experiments, collected the data, analyzed and interpreted the data, and drafted the manuscript. XHS and XJL contributed to data collection and some of the experiments and drafted of the manuscript. ZCX contributed to language polishing. YY and ZYL contributed to the analysis and interpretation of the data. HMX and MZ contributed to the revision of the manuscript. SJQ and HQH contributed to the research design and supervision of the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Chen, Zq., Sun, Xh., Li, Xj. et al. Polydatin attenuates renal fibrosis in diabetic mice through regulating the Cx32-Nox4 signaling pathway. Acta Pharmacol Sin 41, 1587–1596 (2020). https://doi.org/10.1038/s41401-020-0475-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-020-0475-6
Keywords
This article is cited by
-
Natural products targeting ubiquitination to combat kidney fibrosis
Future Journal of Pharmaceutical Sciences (2025)
-
Evaluation of movable tight oil in different reservoir types in the Central Sichuan region
Scientific Reports (2025)
-
Polydatin Mitigates Lead-Induced Nephropathy by Modulating Oxidative Stress, Inflammation, and the AMPK/AKT/Nrf2 Pathway in Rats
Biological Trace Element Research (2025)
-
Resveratrol glycoside inhibits NLRP3/IL-1β/NF-κB to alleviate peritoneal fibrosis in peritoneal dialysis
Clinical and Experimental Nephrology (2025)
-
Comprehensive analysis of pyroptosis-related gene signatures in renal fibrosis
Clinical and Experimental Nephrology (2025)