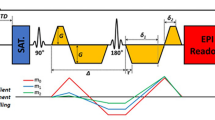
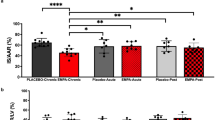
Abstract
Mitochondria are extraordinarily dynamic organelles that have a variety of morphologies, the status of which are controlled by the opposing processes of fission and fusion. Our recent study shows that inhibition of excessive mitochondrial fission by Drp1 inhibitor (Mdivi-1) leads to a reduction in infarct size and left ventricular (LV) dysfunction following cardiac ischemia-reperfusion (I/R) injury in high fat-fed induced pre-diabetic rats. In the present study, we investigated the cardioprotective effects of a mitochondrial fusion promoter (M1) and a combined treatment (M1 and Mdivi-1) in pre-diabetic rats. Wistar rats were given a high-fat diet for 12 weeks to induce prediabetes. The rats then subjected to 30 min-coronary occlusions followed by reperfusion for 120 min. These rats were intravenously administered M1 (2 mg/kg) or M1 (2 mg/kg) combined with Mdivi-1 (1.2 mg/kg) prior to ischemia, during ischemia or at the onset of reperfusion. We showed that administration of M1 alone or in combination with Mdivi-1 prior to ischemia, during ischemia or at the onset of reperfusion all significantly attenuated cardiac mitochondrial ROS production, membrane depolarization, swelling and dynamic imbalance, leading to reduced arrhythmias and infarct size, resulting in improved LV function in pre-diabetic rats. In conclusion, the promotion of mitochondrial fusion at any time-points during cardiac I/R injury attenuated cardiac mitochondrial dysfunction and dynamic imbalance, leading to decreased infarct size and improved LV function in pre-diabetic rats.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
De Lorenzo A, Gratteri S, Gualtieri P, Cammarano A, Bertucci P, Di Renzo L. Why primary obesity is a disease? J Transl Med. 2019;17:169.
Mongraw-Chaffin M, Foster MC, Anderson CAM, Burke GL, Haq N, Kalyani RR, et al. Metabolically healthy obesity, transition to metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol. 2018;71:1857–65.
Kachur S, Lavie CJ, de Schutter A, Milani RV, Ventura HO. Obesity and cardiovascular diseases. Minerva Med. 2017;108:212–28.
Jahangir E, De Schutter A, Lavie CJ. The relationship between obesity and coronary artery disease. Transl Res. 2014;164:336–44.
Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123:92–100.
Hausenloy DJ, Botker HE, Engstrom T, Erlinge D, Heusch G, Ibanez B, et al. Targeting reperfusion injury in patients with st-segment elevation myocardial infarction: trials and tribulations. Eur Heart J. 2017;38:935–41.
Davidson SM, Ferdinandy P, Andreadou I, Botker HE, Heusch G, Ibanez B, et al. Multitarget strategies to reduce myocardial ischemia/reperfusion injury: jacc review topic of the week. J Am Coll Cardiol. 2019;73:89–99.
Hausenloy DJ, Barrabes JA, Botker HE, Davidson SM, Di Lisa F, Downey J, et al. Ischaemic conditioning and targeting reperfusion injury: a 30 year voyage of discovery. Basic Res Cardiol. 2016;111:70.
Feno S, Butera G, Vecellio Reane D, Rizzuto R, Raffaello A. Crosstalk between calcium and ros in pathophysiological conditions. Oxid Med Cell Longev. 2019;2019:9324018.
Ostadal B, Drahota Z, Houstek J, Milerova M, Ostadalova I, Hlavackova M, et al. Developmental and sex differences in cardiac tolerance to ischemia-reperfusion injury: the role of mitochondria (1). Can J Physiol Pharmacol. 2019;97:808–14.
Manning AS, Hearse DJ. Reperfusion-induced arrhythmias: mechanisms and prevention. J Mol Cell Cardiol. 1984;16:497–518.
Hearse DJ, Tosaki A. Free radicals and reperfusion-induced arrhythmias: protection by spin trap agent pbn in the rat heart. Circ Res. 1987;60:375–83.
Tosaki A. Arrhythmogenopharmacotherapy. Front Pharmacol. 2020;11:616.
Zhou T, Prather ER, Garrison DE, Zuo L. Interplay between ROS and antioxidants during ischemia-reperfusion injuries in cardiac and skeletal muscle. Int J Mol Sci. 2018;19:417.
Bertero E, Maack C. Calcium signaling and reactive oxygen species in mitochondria. Circ Res. 2018;122:1460–78.
Koncsos G, Varga ZV, Baranyai T, Boengler K, Rohrbach S, Li L, et al. Diastolic dysfunction in prediabetic male rats: role of mitochondrial oxidative stress. Am J Physiol Heart Circ Physiol. 2016;311:H927–43.
Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–22.
Maneechote C, Palee S, Kerdphoo S, Jaiwongkam T, Chattipakorn SC, Chattipakorn N. Differential temporal inhibition of mitochondrial fission by mdivi-1 exerts effective cardioprotection in cardiac ischemia/reperfusion injury. Clin Sci. 2018;132:1669–83.
Dong Y, Undyala VVR, Przyklenk K. Inhibition of mitochondrial fission as a molecular target for cardioprotection: critical importance of the timing of treatment. Basic Res Cardiol. 2016;111:59.
Ferree A, Shirihai O. Mitochondrial dynamics: the intersection of form and function. Adv Exp Med Biol. 2012;748:13–40.
Whitley BN, Engelhart EA, Hoppins S. Mitochondrial dynamics and their potential as a therapeutic target. Mitochondrion. 2019;49:269–83.
Chan DC. Mitochondrial dynamics and its involvement in disease. Annu Rev Pathol. 2019;15:235–59.
Kornfeld OS, Qvit N, Haileselassie B, Shamloo M, Bernardi P, Mochly-Rosen D. Interaction of mitochondrial fission factor with dynamin related protein 1 governs physiological mitochondrial function in vivo. Sci Rep. 2018;8:14034.
Maneechote C, Palee S, Chattipakorn SC, Chattipakorn N. Roles of mitochondrial dynamics modulators in cardiac ischaemia/reperfusion injury. J Cell Mol Med. 2017;21:2643–53.
Maneechote C, Palee S, Apaijai N, Kerdphoo S, Jaiwongkam T, Chattipakorn SC, et al. Mitochondrial dynamic modulation exerts cardiometabolic protection in obese insulin-resistant rats. Clin Sci. 2019;133:2431–47.
Pei H, Yang Y, Zhao H, Li X, Yang D, Li D, et al. The role of mitochondrial functional proteins in ROS production in ischemic heart diseases. Oxid Med Cell Longev. 2016;2016:5470457.
Maneechote C, Palee S, Kerdphoo S, Jaiwongkam T, Chattipakorn SC, Chattipakorn N. Balancing mitochondrial dynamics via increasing mitochondrial fusion attenuates infarct size and left ventricular dysfunction in rats with cardiac ischemia/reperfusion injury. Clin Sci. 2019;133:497–513.
Sharp WW, Fang YH, Han M, Zhang HJ, Hong Z, Banathy A, et al. Dynamin-related protein 1 (Drp1)-mediated diastolic dysfunction in myocardial ischemia-reperfusion injury: therapeutic benefits of drp1 inhibition to reduce mitochondrial fission. FASEB J. 2014;28:316–26.
Givvimani S, Pushpakumar SB, Metreveli N, Veeranki S, Kundu S, Tyagi SC. Role of mitochondrial fission and fusion in cardiomyocyte contractility. Int J Cardiol. 2015;187:325–33.
Jheng HF, Tsai PJ, Guo SM, Kuo LH, Chang CS, Su IJ, et al. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol Cell Biol. 2012;32:309–19.
Dai W, Jiang L. Dysregulated mitochondrial dynamics and metabolism in obesity, diabetes, and cancer. Front Endocrinol. 2019;10:570.
Maneechote C, Palee S, Kerdphoo S, Jaiwongkam T, Chattipakorn SC, Chattipakorn N. Pharmacological inhibition of mitochondrial fission attenuates cardiac ischemia-reperfusion injury in pre-diabetic rats. Biochem Pharmacol. 2020;182:114295.
Maneechote C, Palee S, Apaijai N, Kerdphoo S, Jaiwongkam T, Chattipakorn SC, et al. Mitochondrial dynamic modulation exerts cardiometabolic protection in obese insulin-resistant rats. Clin Sci. 2019;133:2431–47.
Ding M, Liu C, Shi R, Yu M, Zeng K, Kang J, et al. Mitochondrial fusion promoter restores mitochondrial dynamics balance and ameliorates diabetic cardiomyopathy in an optic atrophy 1-dependent way. Acta Physiol. 2020;229:e13428.
Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, et al. The arrive guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol. 2020;18:e3000410.
National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press (US);2011.
Pratchayasakul W, Kerdphoo S, Petsophonsakul P, Pongchaidecha A, Chattipakorn N, Chattipakorn SC. Effects of high-fat diet on insulin receptor function in rat hippocampus and the level of neuronal corticosterone. Life Sci. 2011;88:619–27.
Thummasorn S, Kumfu S, Chattipakorn S, Chattipakorn N. Granulocyte-colony stimulating factor attenuates mitochondrial dysfunction induced by oxidative stress in cardiac mitochondria. Mitochondrion. 2011;11:457–66.
Apaijai N, Chinda K, Palee S, Chattipakorn S, Chattipakorn N. Combined vildagliptin and metformin exert better cardioprotection than monotherapy against ischemia-reperfusion injury in obese-insulin resistant rats. PLoS One. 2014;9:e102374.
Serasinghe MN, Chipuk JE. Mitochondrial fission in human diseases. Handb Exp Pharmacol. 2017;240:159–88.
Roy S, Kim D, Sankaramoorthy A. Mitochondrial structural changes in the pathogenesis of diabetic retinopathy. J Clin Med. 2019;8:1363.
Ding M, Dong Q, Liu Z, Liu Z, Qu Y, Li X, et al. Inhibition of dynamin-related protein 1 protects against myocardial ischemia-reperfusion injury in diabetic mice. Cardiovasc Diabetol. 2017;16:19.
Lopez-Lluch G. Mitochondrial activity and dynamics changes regarding metabolism in ageing and obesity. Mech Ageing Dev. 2017;162:108–21.
Heo JW, No MH, Park DH, Kang JH, Seo DY, Han J, et al. Effects of exercise on obesity-induced mitochondrial dysfunction in skeletal muscle. Korean J Physiol Pharmacol. 2017;21:567–77.
Mui D, Zhang Y. Mitochondrial scenario: Roles of mitochondrial dynamics in acute myocardial ischemia/reperfusion injury. J Recept Signal Transduct Res. 2021;41:1–5.
Kulek AR, Anzell A, Wider JM, Sanderson TH, Przyklenk K. Mitochondrial quality control: role in cardiac models of lethal ischemia-reperfusion injury. Cells. 2020;9:214.
Siasos G, Tsigkou V, Kosmopoulos M, Theodosiadis D, Simantiris S, Tagkou NM, et al. Mitochondria and cardiovascular diseases-from pathophysiology to treatment. Ann Transl Med. 2018;6:256.
Galan DT, Bito V, Claus P, Holemans P, Abi-Char J, Nagaraju CK, et al. Reduced mitochondrial respiration in the ischemic as well as in the remote nonischemic region in postmyocardial infarction remodeling. Am J Physiol Heart Circ Physiol. 2016;311:H1075–90.
Fakhry M, Skafi N, Fayyad-Kazan M, Kobeissy F, Hamade E, Mebarek S, et al. Characterization and assessment of potential micrornas involved in phosphate-induced aortic calcification. J Cell Physiol. 2018;233:4056–67.
Lesnefsky EJ, Chen Q, Tandler B, Hoppel CL. Mitochondrial dysfunction and myocardial ischemia-reperfusion: implications for novel therapies. Annu Rev Pharmacol Toxicol. 2017;57:535–65.
Zhang W, Siraj S, Zhang R, Chen Q. Mitophagy receptor fundc1 regulates mitochondrial homeostasis and protects the heart from I/R injury. Autophagy. 2017;13:1080–1.
Liu XW, Lu MK, Zhong HT, Wang LH, Fu YP. Panax notoginseng saponins attenuate myocardial ischemia-reperfusion injury through the hif-1α/bnip3 pathway of autophagy. J Cardiovasc Pharmacol. 2019;73:92–9.
Moyzis AG, Sadoshima J, Gustafsson ÅB. Mending a broken heart: the role of mitophagy in cardioprotection. Am J Physiol Heart Circ Physiol. 2015;308:H183–92.
Kubli DA, Gustafsson ÅB. Mitochondria and mitophagy: the yin and yang of cell death control. Circ Res. 2012;111:1208–21.
Feng Y, Madungwe NB, da Cruz Junho CV, Bopassa JC. Activation of g protein-coupled oestrogen receptor 1 at the onset of reperfusion protects the myocardium against ischemia/reperfusion injury by reducing mitochondrial dysfunction and mitophagy. Br J Pharmacol. 2017;174:4329–44.
Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22:1577–90.
Jahani-Asl A, Germain M, Slack RS. Mitochondria: joining forces to thwart cell death. Biochim Biophys Acta. 2010;1802:162–6.
Hoppins S, Nunnari J. Cell biology. Mitochondrial dynamics and apoptosis-the ER connection. Science. 2012;337:1052–4.
Eefting F, Rensing B, Wigman J, Pannekoek WJ, Liu WM, Cramer MJ, et al. Role of apoptosis in reperfusion injury. Cardiovasc Res. 2004;61:414–26.
Kovacs P, Bak I, Szendrei L, Vecsernyes M, Varga E, Blasig IE, et al. Non-specific caspase inhibition reduces infarct size and improves post-ischaemic recovery in isolated ischaemic/reperfused rat hearts. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:501–7.
Mocanu MM, Baxter GF, Yellon DM. Caspase inhibition and limitation of myocardial infarct size: protection against lethal reperfusion injury. Br J Pharmacol. 2000;130:197–200.
Xia Y, Gong KZ, Xu M, Zhang YY, Guo JH, Song Y, et al. Regulation of gap-junction protein connexin 43 by beta-adrenergic receptor stimulation in rat cardiomyocytes. Acta Pharmacol Sin. 2009;30:928–34.
Kohutova J, Elsnicova B, Holzerova K, Neckar J, Sebesta O, Jezkova J, et al. Anti-arrhythmic cardiac phenotype elicited by chronic intermittent hypoxia is associated with alterations in connexin-43 expression, phosphorylation, and distribution. Front Endocrinol. 2018;9:789.
Lampe PD, TenBroek EM, Burt JM, Kurata WE, Johnson RG, Lau AF. Phosphorylation of connexin43 on serine368 by protein kinase c regulates gap junctional communication. J Cell Biol. 2000;149:1503–12.
Yang L, Korge P, Weiss JN, Qu Z. Mitochondrial oscillations and waves in cardiac myocytes: insights from computational models. Biophys J. 2010;98:1428–38.
Meier P, Schirmer SH, Lansky AJ, Timmis A, Pitt B, Seiler C. The collateral circulation of the heart. BMC Med. 2013;11:143.
Toma I, Kim PJ, Dash R, McConnell MV, Nishimura D, Harnish P, et al. Telmisartan in the diabetic murine model of acute myocardial infarction: dual contrast manganese-enhanced and delayed enhancement mri evaluation of the peri-infarct region. Cardiovasc Diabetol. 2016;15:24.
Friedrich MG, Abdel-Aty H, Taylor A, Schulz-Menger J, Messroghli D, Dietz R. The salvaged area at risk in reperfused acute myocardial infarction as visualized by cardiovascular magnetic resonance. J Am Coll Cardiol. 2008;51:1581–7.
Duran JM, Taghavi S, Berretta RM, Makarewich CA, Sharp Iii T, Starosta T, et al. A characterization and targeting of the infarct border zone in a swine model of myocardial infarction. Clin Transl Sci. 2012;5:416–21.
Acknowledgements
This work was supported by the NSTDA Research Chair grant from the National Science and Technology Development Agency Thailand (NC); the Thailand Research Fund grants TRF-Royal Golden Jubilee Program [grant numbers PHD/0144/2558] (CM and NC); the Senior Research Scholar Grant from the National Research Council of Thailand (SCC); and the Chiang Mai University Center of Excellence Award (NC).
Author information
Authors and Affiliations
Contributions
CM performed the experiments, analyzed the data, and wrote the manuscript. SP performed the experiments and analyzed data. SK and TJ performed the experiments. SCC designed the study, contributed to the discussion, and edited the manuscript. NC designed the study, analyzed the data, contributed to the discussion, and edited and finalized the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Maneechote, C., Palee, S., Kerdphoo, S. et al. Modulating mitochondrial dynamics attenuates cardiac ischemia-reperfusion injury in prediabetic rats. Acta Pharmacol Sin 43, 26–38 (2022). https://doi.org/10.1038/s41401-021-00626-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-021-00626-3
Keywords
This article is cited by
-
Sympathetic-like-integrated engineered heart tissue models AGEs-induced adverse remodeling
Cardiovascular Diabetology (2026)
-
Role of mitochondria in physiological activities, diseases, and therapy
Molecular Biomedicine (2025)
-
Chronic hyperglycemia and cardiovascular dysfunction: an in-depth exploration of metabolic and cellular pathways in type 2 diabetes mellitus
Cardiovascular Diabetology – Endocrinology Reports (2025)
-
The Mitochondrial Fusion Promoter M1 Mitigates Cigarette Smoke-Induced Airway Inflammation and Oxidative Stress via the PI3K-AKT Signaling Pathway
Lung (2025)
-
Mitochondrial function in intestinal ischemia-reperfusion injury: mechanisms and therapeutic perspectives
Molecular Biology Reports (2025)