Abstract
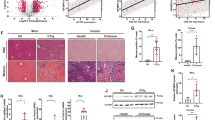
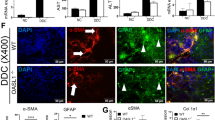
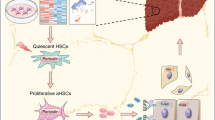
Hepatic stellate cells (HSCs) play an important role in the initiation and development of liver fibrogenesis, and abnormal glucose metabolism is increasingly being considered a crucial factor controlling phenotypic transformation in HSCs. However, the role of the factors affecting glycolysis in HSCs in the experimental models of liver fibrosis has not been completely elucidated. In this study, we showed that glycolysis was significantly enhanced, while the expression of brain and muscle arnt-like protein-1 (Bmal1) was downregulated in fibrotic liver tissues of mice, primary HSCs, and transforming growth factor-β1 (TGF-β1)-induced LX2 cells. Overexpression of Bmal1 in TGF-β1-induced LX2 cells blocked glycolysis and inhibited the proliferation and phenotypic transformation of activated HSCs. We further confirmed the protective effect of Bmal1 in liver fibrosis by overexpressing Bmal1 from hepatic adeno-associated virus 8 in mice. In addition, we also showed that the regulation of glycolysis by Bmal1 is mediated by the isocitrate dehydrogenase 1/α-ketoglutarate (IDH1/α-KG) pathway. Collectively, our results indicated that a novel Bmal1-IDH1/α-KG axis may be involved in regulating glycolysis of activated HSCs and might hence be used as a therapeutic target for alleviating liver fibrosis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Change history
20 May 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41401-021-00685-6
References
Natarajan V, Harris EN, Kidambi S. SECs (Sinusoidal Endothelial Cells), liver microenvironment, and fibrosis. Biomed Res Int. 2017;2017:4097205.
Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–18.
Wang F, Jia Y, Li M, Wang L, Shao J, Guo Q, et al. Blockade of glycolysis-dependent contraction by oroxylin a via inhibition of lactate dehydrogenase-a in hepatic stellate cells. Cell Commun Signal. 2019;17:11.
Lian N, Jin H, Zhang F, Wu L, Shao J, Lu Y, et al. Curcumin inhibits aerobic glycolysis in hepatic stellate cells associated with activation of adenosine monophosphate-activated protein kinase. IUBMB Life. 2016;68:589–96.
Duan L, Ramachandran A, Akakpo JY, Woolbright BL, Zhang Y, Jaeschke H. Mice deficient in pyruvate dehydrogenase kinase 4 are protected against acetaminophen-induced hepatotoxicity. Toxicol Appl Pharmacol. 2020;387:114849.
Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397–411.
Chen Y, Choi SS, Michelotti GA, Chan IS, Swiderska-Syn M, Karaca GF, et al. Hedgehog controls hepatic stellate cell fate by regulating metabolism. Gastroenterology. 2012;143:1319–29 e11.
Mejias M, Gallego J, Naranjo-Suarez S, Ramirez M, Pell N, Manzano A, et al. CPEB4 increases expression of PFKFB3 to induce glycolysis and activate mouse and human hepatic stellate cells, promoting liver fibrosis. Gastroenterology. 2020;159:273–88.
Chang ML, Yang SS. Metabolic signature of hepatic fibrosis: from individual pathways to systems biology. Cells. 2019;8:1423.
Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2:e377.
Asher G, Sassone-Corsi P. Time for food: the intimate interplay between nutrition, metabolism, and the circadian clock. Cell. 2015;161:84–92.
Curtis AM, Bellet MM, Sassone-Corsi P, O'Neill LA. Circadian clock proteins and immunity. Immunity. 2014;40:178–86.
Early JO, Menon D, Wyse CA, Cervantes-Silva MP, Zaslona Z, Carroll RG, et al. Circadian clock protein BMAL1 regulates IL-1beta in macrophages via NRF2. Proc Natl Acad Sci USA. 2018;115:E8460–E8.
Harfmann BD, Schroder EA, Kachman MT, Hodge BA, Zhang X, Esser KA. Muscle-specific loss of Bmal1 leads to disrupted tissue glucose metabolism and systemic glucose homeostasis. Skelet Muscle. 2016;6:12.
Peek CB, Affinati AH, Ramsey KM, Kuo HY, Yu W, Sena LA, et al. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science. 2013;342:1243417.
Deng W, Zhu S, Zeng L, Liu J, Kang R, Yang M, et al. The circadian clock controls immune checkpoint pathway in sepsis. Cell Rep. 2018;24:366–78.
Wang B, Ye Y, Yang X, Liu B, Wang Z, Chen S, et al. SIRT2-dependent IDH1 deacetylation inhibits colorectal cancer and liver metastases. EMBO Rep. 2020;21:e48183.
Suzuki J, Yamada T, Inoue K, Nabe S, Kuwahara M, Takemori N, et al. The tumor suppressor menin prevents effector CD8 T-cell dysfunction by targeting mTORC1-dependent metabolic activation. Nat Commun. 2018;9:3296.
Xiang S, Gu H, Jin L, Thorne RF, Zhang XD, Wu M. LncRNA IDH1-AS1 links the functions of c-Myc and HIF1alpha via IDH1 to regulate the Warburg effect. Proc Natl Acad Sci USA. 2018;115:E1465–E74.
Wen YA, Xiong X, Scott T, Li AT, Wang C, Weiss HL, et al. The mitochondrial retrograde signaling regulates Wnt signaling to promote tumorigenesis in colon cancer. Cell Death Differ. 2019;26:1955–69.
Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci USA. 2008;105:15172–7.
Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–31.
Sharma A, Kumar V. Metabolic plasticity mediates differential responses to spring and autumn migrations: Evidence from gene expression patterns in migratory buntings. Exp Physiol. 2019;104:1841–57.
Zhou L, Wang Z, Hu C, Zhang C, Kovatcheva-Datchary P, Yu D, et al. Integrated metabolomics and lipidomics analyses reveal metabolic reprogramming in human glioma with IDH1 mutation. J Proteome Res. 2019;18:960–9.
Fujiwara H, Tateishi K, Misumi K, Hayashi A, Igarashi K, Kato H, et al. Mutant IDH1 confers resistance to energy stress in normal biliary cells through PFKP-induced aerobic glycolysis and AMPK activation. Sci Rep. 2019;9:18859.
Chen C, Shi Y, Li Y, He ZC, Zhou K, Zhang XN, et al. A glycolysis-based ten-gene signature correlates with the clinical outcome, molecular subtype and IDH1 mutation in glioblastoma. J Genet Genom. 2017;44:519–30.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (Grant/Award Number: 82070629) and Natural Science Foundation of Anhui Province of China (Grant/Award Number: 20080852MH242).
Author information
Authors and Affiliations
Contributions
LX, YWZ and MFW conceived and designed the study, performed the experiments, collected the data, analyzed and interpreted the data, and drafted the manuscript. JS and JLC contributed to data collection and some of the experiments. JQW, QXL and SYC provided a series of experimental instructions. LZ contributed to language polishing. TYY contributed to the revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Xu, L., Yang, Ty., Zhou, Yw. et al. Bmal1 inhibits phenotypic transformation of hepatic stellate cells in liver fibrosis via IDH1/α-KG-mediated glycolysis. Acta Pharmacol Sin 43, 316–329 (2022). https://doi.org/10.1038/s41401-021-00658-9
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-021-00658-9
Keywords
This article is cited by
-
Prolyl 4-hydroxylase subunit alpha-2 acts as a TRIM21 ubiquitination substrate to promote papillary thyroid cancer progression via the glycolytic pathway
Cell Death & Disease (2025)
-
Focal adhesion kinase promotes aerobic glycolysis in hepatic stellate cells via the cyclin D1/c-Myc/MCT-1 pathway to induce liver fibrosis
Scientific Reports (2025)
-
The m6A reader IGF2BP2 regulates glycolytic metabolism and mediates histone lactylation to enhance hepatic stellate cell activation and liver fibrosis
Cell Death & Disease (2024)
-
Metagenomic comparison of intestinal microbiota between normal and liver fibrotic rhesus macaques (Macaca mulatta)
Scientific Reports (2024)
-
Mitochondrial heterogeneity in diseases
Signal Transduction and Targeted Therapy (2023)