Abstract
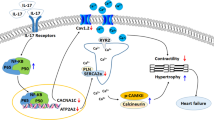
Interleukin-17A (IL-17), a potent proinflammatory cytokine, has been shown to participate in cardiac electrical disorders. Diabetes mellitus is an independent risk factor for ventricular arrhythmia. In this study, we investigated the role of IL-17 in ventricular arrhythmia of diabetic mice. Diabetes was induced in both wild-type and IL-17 knockout mice by intraperitoneal injection of streptozotocin (STZ). High-frequency electrical stimuli were delivered into the right ventricle to induce ventricular arrhythmias. We showed that the occurrence rate of ventricular tachycardia was significantly increased in diabetic mice, which was attenuated by IL-17 knockout. We conducted optical mapping on perfused mouse hearts and found that cardiac conduction velocity (CV) was significantly decreased, and action potential duration (APD) was prolonged in diabetic mice, which were mitigated by IL-17 knockout. We performed whole-cell patch clamp recordings from isolated ventricular myocytes, and found that the densities of Ito, INa and ICa,L were reduced, the APDs at 50% and 90% repolarization were increased, and early afterdepolarization (EAD) was markedly increased in diabetic mice. These alterations were alleviated by the knockout of IL-17. Moreover, knockout of IL-17 alleviated the downregulation of Nav1.5 (the pore forming subunit of INa), Cav1.2 (the main component subunit of ICa,L) and KChIP2 (potassium voltage-gated channel interacting protein 2, the regulatory subunit of Ito) in the hearts of diabetic mice. The expression of NF-κB was significantly upregulated in the hearts of diabetic mice, which was suppressed by IL-17 knockout. In neonatal mouse ventricular myocytes, knockdown of NF-κB significantly increased the expression of Nav1.5, Cav1.2 and KChIP2. These results imply that IL-17 may represent a potential target for the development of agents against diabetes-related ventricular arrhythmias.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Weidner K, Behnes M, Schupp T, Rusnak J, Reiser L, Bollow A, et al. Type 2 diabetes is independently associated with all-cause mortality secondary to ventricular tachyarrhythmias. Cardiovasc Diabetol. 2018;17:125.
Priori SG, Blomstrom-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Europace 2015;17:v319.
Jouven X, Lemaître RN, Rea TD, Sotoodehnia N, Empana J. Siscovick D S. Diabetes, glucose level, and risk of sudden cardiac death. Eur Heart J. 2005;26:2142–7.
Grisanti LA. Diabetes and arrhythmias: pathophysology, mechanisms and therapeutic outcomes. Front Physiol. 2018;9:1669.
Pacher U, Nanasi K. Electrophysiological changes in rat ventricular and atrial myocardium at different stages of experimental diabetes. Acta Physiol (Oxf). 1999;166:7–13.
Lu Z, Jiang YP, Wu CYC, Ballou LM, Liu S, Carpenter ES, et al. Increased persistent sodium current due to decreased PI3K signaling contributes to QT prolongation in the diabetic heart. Diabetes. 2013;62:4257–65.
Sato T, Kobayashi T, Kuno A, Miki T, Tanno M, Kouzu H, et al. Type 2 diabetes induces subendocardium-predominant reduction in transient outward K+ current with downregulation of Kv4.2 and KChIP2. Am J Physiol-Heart C. 2014;306:H1054–65.
Erickson JR, Pereira L, Wang L, Han G, Ferguson A, Dao K, et al. Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature. 2013;502:372–6.
El Khoury N, Mathieu S, Fiset C. Interleukin-1β reduces L-type Ca2+ current through protein kinase Cϵ activation in mouse heart. J Biol Chem. 2014;289:21896–908.
Monnerat G, Alarcón ML, Vasconcellos LR, Hochman-Mendez C, Brasil G, Bassani RA, et al. Macrophage-dependent IL-1β production induces cardiac arrhythmias in diabetic mice. Nat Commun. 2016;7:13344.
Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity 2011;34:149–62.
Song X, Qian Y. IL-17 family cytokines mediated signaling in the pathogenesis of inflammatory diseases. Cell Signal. 2013;25:2335–47.
Liao Y, Xia N, Zhou S, Tang T, Yan X, Lv B, et al. Interleukin-17A contributes to myocardial ischemia/reperfusion injury by regulating cardiomyocyte apoptosis and neutrophil infiltration. J Am Coll Cardiol. 2012;59:420–9.
Robert M, Miossec P. Effects of interleukin 17 on the cardiovascular system. Autoimmun Rev. 2017;16:984–91.
Fu X, Zhao N, Dong Q, Du L, Chen X, Wu Q, et al. Interleukin-17A contributes to the development of post-operative atrial fibrillation by regulating inflammation and fibrosis in rats with sterile pericarditis. Int J Mol Med. 2015;36:83–92.
Baldeviano GC, Barin JG, Talor MV, Srinivasan S, Bedja D, Zheng D, et al. Interleukin-17A is dispensable for myocarditis but essential for the progression to dilated cardiomyopathy. Circ Res. 2010;106:1646–55.
Zhou SF, Yuan J, Liao MY, Xia N, Tang TT, Li JJ, et al. IL-17A promotes ventricular remodeling after myocardial infarction. J Mol Med (Berl). 2014;92:1105–16.
Zhang Y, Zhang Y, Li T, Wang J, Jiang Y, Zhao Y, et al. Ablation of interleukin-17 alleviated cardiac interstitial fibrosis and improved cardiac function via inhibiting long non-coding RNA-AK081284 in diabetic mice. J Mol Cell Cardiol. 2018;115:64–72.
Zhang Y, Sun L, Xuan L, Pan Z, Hu X, Liu H, et al. Long non-coding RNA CCRR controls cardiac conduction via regulating intercellular coupling. Nat Commun. 2018;9:4176.
Lang D, Sulkin M, Lou Q, Efimov IR. Optical mapping of action potentials and calcium transients in the mouse heart. J Vis Exp. 2011;55:e3275.
Stables CL, Musa H, Mitra A, Bhushal S, Deo M, Guerrero-Serna G, et al. Reduced Na+ current density underlies impaired propagation in the diabetic rabbit ventricle. J Mol Cell Cardiol. 2014;69:24–31.
Shang LL, Sanyal S, Pfahnl AE, Jiao Z, Allen J, Liu H, et al. NF-kappaB-dependent transcriptional regulation of the cardiac scn5a sodium channel by angiotensin II. Am J Physiol Cell Physiol. 2008;294:C372–9.
Liu W, Wang G, Zhang C, Ding W, Cheng W, Luo Y, et al. MG53, a novel regulator of KChIP2 and Ito,f, plays a critical role in electrophysiological remodeling in cardiac hypertrophy. Circulation. 2019;139:2142–56.
Panama BK, Latour-Villamil D, Farman GP, Zhao D, Bolz S, Kirshenbaum LA, et al. Nuclear factor κB downregulates the transient outward potassium current Ito,f through control of KChIP2 expression. Circ Res. 2011;108:537–43.
Rolim N, Skårdal K, Høydal M, Sousa MML, Malmo V, Kaurstad G, et al. Aerobic interval training reduces inducible ventricular arrhythmias in diabetic mice after myocardial infarction. Basic Res Cardiol. 2015;110:44.
Rajab M, Jin H, Welzig CM, Albano A, Aronovitz M, Zhang Y, et al. Increased inducibility of ventricular tachycardia and decreased heart rate variability in a mouse model for type 1 diabetes: effect of pravastatin. Am J Physiol-Heart C. 2013;305:H1807–16.
Lakin R, Polidovitch N, Yang S, Guzman C, Gao X, Wauchop M, et al. Inhibition of soluble TNFα prevents adverse atrial remodeling and atrial arrhythmia susceptibility induced in mice by endurance exercise. J Mol Cell Cardiol. 2019;129:165–73.
Arababadi MK, Nosratabadi R, Hassanshahi G, Yaghini N, Pooladvand V, Shamsizadeh A, et al. Nephropathic complication of type-2 diabetes is following pattern of autoimmune diseases?. Diabetes Res Clin Pr. 2010;87:33–7.
Chang S, Hsiao Y, Tsai Y, Lin S, Liu S, Lin Y, et al. Interleukin-17 enhances cardiac ventricular remodeling via activating MAPK pathway in ischemic heart failure. J Mol Cell Cardiol. 2018;122:69–79.
Meo M, Meste O, Signore S, Sorrentino A, Cannata A, Zhou Y, et al. Reduction in Kv current enhances the temporal dispersion of the action potential in diabetic myocytes: insights from a novel repolarization algorithm. J Am Heart Assoc. 2016;5:e003078.
Niwa N, Nerbonne JM. Molecular determinants of cardiac transient outward potassium current (Ito) expression and regulation. J Mol Cell Cardiol. 2010;48:12–25.
Foeger NC, Marionneau C, Nerbonne JM. Co-assembly of Kv4 α subunits with K+ channel-interacting protein 2 stabilizes protein expression and promotes surface retention of channel complexes. J Biol Chem. 2010;285:33413–22.
Nattel S, Maguy A, Le Bouter S, Yeh Y. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev. 2007;87:425–56.
Nishiyama A, Ishii DN, Backx PH, Pulford BE, Birks BR, Tamkun MM. Altered K+ channel gene expression in diabetic rat ventricle: isoform switching between Kv4.2 and Kv1.4. Am J Physiol-Heart C. 2001;281:H1800–7.
Axelsen LN, Calloe K, Braunstein TH, Riemann M, Hofgaard JP, Liang B, et al. Diet-induced pre-diabetes slows cardiac conductance and promotes arrhythmogenesis. Cardiovasc Diabetol. 2015;14:87.
Shimoni Y, Emmett T, Schmidt R, Nygren A, Kargacin G. Sex-dependent impairment of cardiac action potential conduction in type 1 diabetic rats. Am J Physiol-Heart C. 2009;296:H1442–50.
Bohne LJ, Johnson D, Rose RA, Wilton SB, Gillis AM. The association between diabetes mellitus and atrial fibrillation: clinical and mechanistic insights. Front Physiol. 2019;10:135.
Cominacini L, Anselmi M, Garbin U, Fratta Pasini A, Stranieri C, Fusaro M, et al. Enhanced plasma levels of oxidized low-density lipoprotein increase circulating nuclear factor-kappa B activation in patients with unstable angina. J Am Coll Cardiol. 2005;46:799–806.
Timmers L, van Keulen JK, Hoefer IE, Meijs MFL, van Middelaar B, den Ouden K, et al. Targeted deletion of nuclear factor κB p50 enhances cardiac remodeling and dysfunction following myocardial infarction. Circ Res. 2009;104:699–706.
Shembade N, Harhaj EW. IKKi: a novel regulator of Act1, IL-17 signaling and pulmonary inflammation. Cell Mol Immunol. 2011;8:447–9.
Mcdaniel DK, Eden K, Ringel VM, Allen IC. Emerging roles for noncanonical NF-κB signaling in the modulation of inflammatory bowel disease pathobiology. Inflamm Bowel Dis. 2016;22:2265–79.
Baldwin AS. The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–81.
Narayanan D, Xi Q, Pfeffer LM, Jaggar JH. Mitochondria control functional CaV 1.2 expression in smooth muscle cells of cerebral arteries. Circ Res. 2010;107:631–41.
Acknowledgements
This work was supported by National Key R&D Program of China (2017YFC1307404 to ZP), National Natural Science Foundation of China (82070344, 81870295 to ZP 81861128022 to BY), Heilongjiang Touyan Innovation Team Program and CAMS Innovation Fund for Medical Sciences (CIFMS), 2019-I2M-5-078 (to BY).
Author information
Authors and Affiliations
Contributions
DSL and GLX performed experiments, analyzed data, and prepared the paper. JMY, CZL, RXZ, TT, ZL, KWS, YG, XNL, and JW helped perform experiments and collect data. YJL and ZWP designed the project, oversaw the experiments and prepared the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Li, Ds., Xue, Gl., Yang, Jm. et al. Knockout of interleukin-17A diminishes ventricular arrhythmia susceptibility in diabetic mice via inhibiting NF-κB-mediated electrical remodeling. Acta Pharmacol Sin 43, 307–315 (2022). https://doi.org/10.1038/s41401-021-00659-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-021-00659-8
Keywords
This article is cited by
-
Recent advances in understanding the roles of T cells in atrial fibrillation
npj Cardiovascular Health (2024)
-
Forced activation of dystrophin transcription by CRISPR/dCas9 reduced arrhythmia susceptibility via restoring membrane Nav1.5 distribution
Gene Therapy (2023)