Abstract
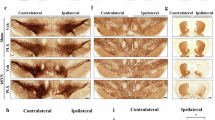
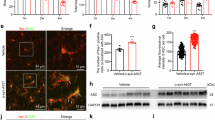
Neuroinflammation plays an important role in neurodegenerative diseases, such as Parkinson’s disease (PD) and Alzheimer’s disease. HACE1 (HECT domain and Ankyrin repeat Containing E3 ubiquitin-protein ligase 1) is a tumor suppressor. Recent evidence suggests that HACE1 may be involved in oxidative stress responses. Due to the critical role of ROS in neuroinflammation, we speculated that HACE1 might participate in neuroinflammation and related neurodegenerative diseases, such as PD. In this study, we investigated the role of HACE1 in neuroinflammation of PD models. We showed that HACE1 knockdown exacerbated LPS-induced neuroinflammation in BV2 microglial cells in vitro through suppressing ubiquitination and degradation of activated Rac1, an NADPH oxidase subunit. Furthermore, we showed that HACE1 exerted vital neuronal protection through increasing Rac1 activity and stability in LPS-treated SH-SY5Y cells, as HACE1 knockdown leading to lower tolerance to LPS challenge. In MPTP-induced acute PD mouse model, HACE1 knockdown exacerbated motor deficits by activating Rac1. Finally, mutant α-synuclein (A53T)-overexpressing mice, a chronic PD mouse model, exhibited age-dependent reduction of HACE1 levels in the midbrain and striatum, implicating that HACE1 participated in PD pathological progression. This study for the first time demonstrates that HACE1 is a negative regulator of neuroinflammation and involved in the PD pathogenesis by regulating Rac1 activity. The data support HACE1 as a potential target for PD and other neurodegenerative diseases.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Samii A, Nutt JG, Ransom BR. Parkinson’s disease. Lancet. 2004;363:1783–93.
Ha D, Stone DK, Mosley RL, Gendelman HE. Immunization strategies for Parkinson’s disease. Parkinsonism Relat D. 2012;18:S218–S221.
Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol. 2009;8:382–97.
Damier P, Hirsch EC, Zhang P, Agid Y, Javoy-Agid F. Glutathione peroxidase, glial cells and Parkinson’s disease. Neuroscience. 1993;52:1–6.
Ouchi Y, Yoshikawa E, Sekine Y, Futatsubashi M, Kanno T, Ogusu T, et al. Microglial activation and dopamine terminal loss in early Parkinson’s disease. Ann Neurol. 2005;57:168–75.
Linnerbauer M, Wheeler MA, Quintana FJ. Astrocyte crosstalk in CNS inflammation. Neuron. 2020;108:608–22.
Yang QQ, Zhou JW. Neuroinflammation in the central nervous system: symphony of glial cells. Glia. 2019;67:1017–35.
Imamura K, Hishikawa N, Sawada M, Nagatsu T, Yoshida M, Hashizume Y. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol. 2003;106:518–26.
Cetinbas N, Daugaard M, Mullen AR, Hajee S, Rotblat B, Lopez A, et al. Loss of the tumor suppressor Hace1 leads to ROS-dependent glutamine addiction. Oncogene. 2015;34:4005–10.
Daugaard M, Nitsch R, Razaghi B, McDonald L, Jarrar A, Torrino S, et al. Hace1 controls ROS generation of vertebrate Rac1-dependent NADPH oxidase complexes. Nat Commun. 2013;4:2180.
Razaghi B, Steele SL, Prykhozhij SV, Stoyek MR, Hill JA, Cooper MD, et al. Hace1 influences zebrafish cardiac development via ROS-dependent mechanisms. Dev Dyn. 2018;247:289–303.
Chiurchiù V, Maccarrone M. Chronic inflammatory disorders and their redox control: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2011;15:2605–41.
Lee IT, Yang CM. Role of NADPH oxidase/ROS in pro-inflammatory mediators-induced airway and pulmonary diseases. Biochem Pharmacol. 2012;84:581–90.
Yan ZQ, Gibson SA, Buckley JA, Qin HW, Benveniste EN. Role of the JAK/STAT signaling pathway in regulation of innate immunity in neuroinflammatory diseases. Clin Immunol. 2018;189:4–13.
Qin HW, Buckley JA, Li XR, Liu YD, Fox TH, Meares GP, et al. Inhibition of the JAK/STAT pathway protects against α-synuclein-induced neuroinflammation and dopaminergic neurodegeneration. J Neurosci. 2016;36:5144–59.
Yauger YJ, Bermudez S, Moritz KE, Glaser E, Stoica B, Byrnes KR. Iron accentuated reactive oxygen species release by NADPH oxidase in activated microglia contributes to oxidative stress in vitro. J Neuroinflammation. 2019;16:41.
Qin LY, Liu YX, Wang TG, Wei SJ, Block ML, Wilson B, et al. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J Biol Chem. 2004;279:1415–21.
Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313.
Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–77.
Goka ET, Lippman ME. Loss of the E3 ubiquitin ligase HACE1 results in enhanced Rac1 signaling contributing to breast cancer progression. Oncogene. 2015;34:5395–405.
Mettouchi A, Lemichez E. Ubiquitylation of active Rac1 by the E3 ubiquitin-ligase HACE1. Small GTPases. 2012;3:102–6.
Hurley LL, Tizabi Y. Neuroinflammation, neurodegeneration, and depression. Neurotox Res. 2013;23:131–44.
Kempuraj D, Thangavel R, Selvakumar GP, Zaheer S, Ahmed ME, Raikwar SP, et al. Brain and peripheral atypical inflammatory mediators potentiate neuroinflammation and neurodegeneration. Front Cell Neurosci. 2017;11:216.
Perry VH, Teeling J. Microglia and macrophages of the central nervous system: the contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin Immunopathol. 2013;35:601–12.
Schain M, Kreisl WC. Neuroinflammation in neurodegenerative disorders-a review. Curr Neurol Neurosci Rep. 2017;17:25.
Cetinbas N, Daugaard M, Mullen AR, Hajee S, Rotblat B, Lopez A, et al. Loss of the tumor suppressor Hace1 leads to ROS-dependent glutamine addiction. Oncogene. 2015;34:4005–10.
Kumar B, Roy A, Asha K, Walia NS, Ansari MA, Chandran B. HACE1, an E3 ubiquitin protein ligase, mitigates Kaposi’s Sarcoma-associated herpesvirus infection-induced oxidative stress by promoting Nrf2 activity. J Virol. 2019;93:e01812–01818.
Milton VJ, Sweeney ST. Oxidative stress in synapse development and function. Dev Neurobiol. 2012;72:100–10.
Bischoff LJM, Kuijper IA, Schimming JP, Wolters L, Braak BT, Langenberg JP, et al. A systematic analysis of Nrf2 pathway activation dynamics during repeated xenobiotic exposure. Arch Toxicol. 2019;93:435–51.
Lam GY, Huang J, Brumell JH. The many roles of NOX2 NADPH oxidase-derived ROS in immunity. Semin Immunopathol. 2010;32:415–30.
Fernandez-Marcos PJ, Nóbrega-Pereira S. NADPH: new oxygen for the ROS theory of aging. Oncotarget. 2016;7:50814–5.
Zhang LY, Chen X, Sharma P, Moon M, Sheftel AD, Dawood F, et al. HACE1-dependent protein degradation provides cardiac protection in response to haemodynamic stress. Nat Commun. 2014;5:3430.
Acosta MI, Urbach S, Doye A, Ng YW, Boudeau J, Mettouchi A, et al. Group-I PAKs-mediated phosphorylation of HACE1 at serine 385 regulates its oligomerization state and Rac1 ubiquitination. Sci Rep. 2018;8:1410.
Rathinam R, Berrier A, Alahari SK. Role of Rho GTPases and their regulators in cancer progression. Front Biosci. 2011;16:2561–71.
Kempuraj D, Thangavel R, Natteru PA, Selvakumar GP, Saeed D, Zahoor H, et al. Neuroinflammation induces neurodegeneration. J Neurol Neurosurg Spine. 2016;1:1003.
Tansey MG, Goldberg MS. Neuroinflammation in Parkinson’s disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis. 2010;37:510–8.
Lawrimore CJ, Coleman LG, Zou J, Crews FT. Ethanol induction of innate immune signals across BV2 microglia and SH-SY5Y neuroblastoma involves induction of IL-4 and IL-13. Brain Sci. 2019;9:228.
Park J, Lim CS, Seo H, Park CA, Zhuo M, Kaang BK, et al. Pain perception in acute model mice of Parkinson’s disease induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Mol Pain. 2015;11:28.
Acknowledgements
The study was supported by grants from Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (No. 2016-I2M-3-011), Chinese Academy of Medical Sciences Fundamental Research Funds for the Central Universities (No. 2018RC350002), National Natural Science Foundation of China (No. 81630097, 81773718, 21772235).
Author information
Authors and Affiliations
Contributions
CXZ and DZ designed the study and drafted the manuscript. CXZ, LW, HYY, JMS carried out behavioral tests, immunohistochemistry and Western blot assay. HL, ZHZ, CJ, FYY and FYL participated in the Western blot assay. XQB revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Zang, Cx., Wang, L., Yang, Hy. et al. HACE1 negatively regulates neuroinflammation through ubiquitylating and degrading Rac1 in Parkinson’s disease models. Acta Pharmacol Sin 43, 285–294 (2022). https://doi.org/10.1038/s41401-021-00778-2
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-021-00778-2
Keywords
This article is cited by
-
Immune cell metabolic dysfunction in Parkinson’s disease
Molecular Neurodegeneration (2025)
-
Shared interactions of six neurotropic viruses with 38 human proteins: a computational and literature-based exploration of viral interactions and hijacking of human proteins in neuropsychiatric disorders
Discover Mental Health (2025)
-
Expression of USP25 associates with fibrosis, inflammation and metabolism changes in IgG4-related disease
Nature Communications (2024)
-
JAK-STAT signaling in inflammation and stress-related diseases: implications for therapeutic interventions
Molecular Biomedicine (2023)
-
Key role of Rho GTPases in motor disorders associated with neurodevelopmental pathologies
Molecular Psychiatry (2023)