Abstract
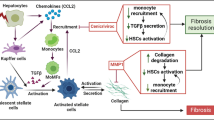
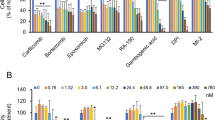
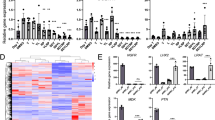
Liver fibrosis is the common consequence of almost all liver diseases and has become an urgent clinical problem without efficient therapies. Recent evidence has shown that hepatocytes-derived extracellular vesicles (EVs) play important roles in liver pathophysiology, but little is known about the role of damaged hepatocytes-derived EVs in hepatic stellate cell (HSC) activation and following fibrosis. Tetramethylpyrazine (TMP) from Ligusticum wallichii Franchat exhibits a broad spectrum of biological activities including liver protection. In this study, we investigated whether TMP exerted liver-protective action through regulating EV-dependent intercellular communication between hepatocytes and HSCs. Chronic liver injury was induced in mice by CCl4 (1.6 mg/kg, i.g.) twice a week for 8 weeks. In the last 4 weeks of CCl4 administration, mice were given TMP (40, 80, 160 mg·kg−1·d−1, i.g.). Acute liver injury was induced in mice by injection of a single dose of CCl4 (0.8 mg/kg, i.p.). After injection, mice were treated with TMP (80 mg/kg) every 24 h. We showed that TMP treatment dramatically ameliorated CCl4-induced oxidative stress and hepatic inflammation as well as acute or chronic liver fibrosis. In cultured mouse primary hepatocytes (MPHs), treatment with CCl4 or acetaminophen resulted in mitochondrial dysfunction, release of mitochondrial DNA (mtDNA) from injured hepatocytes to adjacent hepatocytes and HSCs through EVs, mediating hepatocyte damage and fibrogenic responses in activated HSCs; pretreatment of MPHs with TMP (25 μM) prevented all these pathological effects. Transplanted serum EVs from TMP-treated mice prevented both initiation and progression of liver fibrosis caused by CCl4. Taken together, this study unravels the complex mechanisms underlying the protective effects of TMP against mtDNA-containing EV-mediated hepatocyte injury and HSC activation during liver injury, and provides critical evidence inspiring the development of TMP-based innovative therapeutic agents for the treatment of liver fibrosis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Li Y, Liu R, Wu J, Li X. Self-eating: friend or foe? The emerging role of autophagy in fibrotic diseases. Theranostics. 2020;10:7993–8017.
Hirsova P, Ibrahim SH, Verma VK, Morton LA, Shah VH, LaRusso NF, et al. Extracellular vesicles in liver pathobiology: small particles with big impact. Hepatology. 2016;64:2219–33.
Liu R, Li X, Zhu W, Wang Y, Zhao D, Wang X, et al. Cholangiocyte-derived exosomal long noncoding RNA H19 promotes hepatic stellate cell activation and cholestatic liver fibrosis. Hepatology. 2019;70:1317–35.
Dasgupta D, Nakao Y, Mauer AS, Thompson JM, Sehrawat TS, Liao CY, et al. IRE1A stimulates hepatocyte-derived extracellular vesicles that promote inflammation in mice with steatohepatitis. Gastroenterology. 2020;159:1487–503.e17.
Jiang F, Chen Q, Wang W, Ling Y, Yan Y, Xia P. Hepatocyte-derived extracellular vesicles promote endothelial inflammation and atherogenesis via microRNA-1. J Hepatol. 2020;72:156–66.
Povero D, Panera N, Eguchi A, Johnson CD, Papouchado BG, de Araujo Horcel L, et al. Lipid-induced hepatocyte-derived extracellular vesicles regulate hepatic stellate cell via microRNAs targeting PPAR-γ. Cell Mol Gastroenterol Hepatol. 2015;1:646–63.e4.
An P, Wei LL, Zhao S, Sverdlov DY, Vaid KA, Miyamoto M, et al. Hepatocyte mitochondria-derived danger signals directly activate hepatic stellate cells and drive progression of liver fibrosis. Nat Commun. 2020;11:2362.
Chen L, Chen R, Kemper S, Brigstock DR. Pathways of production and delivery of hepatocyte exosomes. J Cell Commun Signal. 2018;12:343–57.
Li X, Chen R, Kemper S, Brigstock DR. Extracellular vesicles from hepatocytes are therapeutic for toxin-mediated fibrosis and gene expression in the liver. Front Cell Dev Biol. 2019;7:368.
Dansako H, Ueda Y, Satoh S, Kato N. Extracellular vesicles activate ATM-Chk2 signaling pathway through the intercellular transfer of mitochondrial DNA in HBV-infected human hepatocytes. FASEB J. 2021;35:e21680.
Zhao S, Zhang Z, Yao Z, Shao J, Chen A, Zhang F, et al. Tetramethylpyrazine attenuates sinusoidal angiogenesis via inhibition of hedgehog signaling in liver fibrosis. IUBMB Life. 2017;69:115–27.
Gao B, Lin X, Jing H, Fan J, Ji C, Jie Q, et al. Local delivery of tetramethylpyrazine eliminates the senescent phenotype of bone marrow mesenchymal stromal cells and creates an anti-inflammatory and angiogenic environment in aging mice. Aging Cell. 2018;17:e12741.
Zhang F, Lu S, He J, Jin H, Wang F, Wu L, et al. Ligand activation of PPARγ by ligustrazine suppresses pericyte functions of hepatic stellate cells via SMRT-mediated transrepression of HIF-1α. Theranostics. 2018;8:610–26.
Cai Y, Xu B, Zhou F, Wu J, Li S, Zheng Q, et al. Si-Ni-San ameliorates chronic colitis by modulating type I interferons-mediated inflammation. Phytomedicine. 2021;84:153495.
Li X, Liu R, Yang J, Sun L, Zhang L, Jiang Z, et al. The role of long noncoding RNA H19 in gender disparity of cholestatic liver injury in multidrug resistance 2 gene knockout mice. Hepatology. 2017;66:869–84.
Mailloux RJ, Treberg JR. Protein S-glutathionlyation links energy metabolism to redox signaling in mitochondria. Redox Biol. 2016;8:110–8.
Li X, Liu R, Wang Y, Zhu W, Zhao D, Wang X, et al. Cholangiocyte-derived exosomal lncRNA H19 promotes macrophage activation and hepatic inflammation under cholestatic conditions. Cells. 2020;9:190.
Bock FJ, Tait SWG. Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol. 2020;21:85–100.
Ruart M, Chavarria L, Campreciós G, Suárez-Herrera N, Montironi C, Guixé-Muntet S, et al. Impaired endothelial autophagy promotes liver fibrosis by aggravating the oxidative stress response during acute liver injury. J Hepatol. 2019;70:458–69.
Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–7.
Riley JS, Quarato G, Cloix C, Lopez J, O'Prey J, Pearson M, et al. Mitochondrial inner membrane permeabilisation enables mtDNA release during apoptosis. EMBO J. 2018;37:37.
Dong J, Viswanathan S, Adami E, Singh BK, Chothani SP, Ng B, et al. Hepatocyte-specific IL11 cis-signaling drives lipotoxicity and underlies the transition from NAFLD to NASH. Nat Commun. 2021;12:66.
Saikia M, Nath R, Devi D. Genetic diversity and phylogeny analysis of Antheraea assamensis Helfer (Lepidoptera: Saturniidae) based on mitochondrial DNA sequences. J Genet. 2019;98:98.
Mooring M, Fowl BH, Lum S, Liu Y, Yao K, Softic S, et al. Hepatocyte stress increases expression of yes-associated protein and transcriptional coactivator with PDZ-binding motif in hepatocytes to promote parenchymal inflammation and fibrosis. Hepatology. 2020;71:1813–30.
Ge X, Arriazu E, Magdaleno F, Antoine DJ, Dela Cruz R, Theise N, et al. High mobility group box-1 drives fibrosis progression signaling via the receptor for advanced glycation end products in mice. Hepatology. 2018;68:2380–404.
Zhang F, Jin H, Wu L, Shao J, Wu X, Lu Y, et al. Ligustrazine disrupts lipopolysaccharide-activated NLRP3 inflammasome pathway associated with inhibition of Toll-like receptor 4 in hepatocytes. Biomed Pharmacother. 2016;78:204–9.
Roehlen N, Crouchet E, Baumert TF. Liver fibrosis: mechanistic concepts and therapeutic perspectives. Cells. 2020;9:9.
Konerman MA, Jones JC, Harrison SA. Pharmacotherapy for NASH: current and emerging. J Hepatol. 2018;68:362–75.
Zhang Y, Jiang M, Cui BW, Jin CH, Wu YL, Shang Y, et al. P2X7 receptor-targeted regulation by tetrahydroxystilbene glucoside in alcoholic hepatosteatosis: a new strategy towards macrophage-hepatocyte crosstalk. Br J Pharmacol. 2020;177:2793–811.
Hu J, Cao G, Wu X, Cai H, Cai B. Tetramethylpyrazine inhibits activation of hepatic stellate cells through hedgehog signaling pathways in vitro. Biomed Res Int. 2015;2015:603067.
Ma X, Ruan Q, Ji X, Yang J, Peng H. Ligustrazine alleviates cyclophosphamide-induced hepatotoxicity via the inhibition of Txnip/Trx/NF-kappaB pathway. Life Sci. 2021;274:119331.
Luangmonkong T, Suriguga S, Mutsaers H, Groothuis G, Olinga P, Boersema M. Targeting oxidative stress for the treatment of liver fibrosis. Rev Physiol Biochem Pharmacol. 2018;175:71–102.
Gong X, Ivanov VN, Davidson MM, Hei TK. Tetramethylpyrazine (TMP) protects against sodium arsenite-induced nephrotoxicity by suppressing ROS production, mitochondrial dysfunction, pro-inflammatory signaling pathways and programed cell death. Arch Toxicol. 2015;89:1057–70.
Kim J, Gupta R, Blanco LP, Yang S, Shteinfer-Kuzmine A, Wang K, et al. VDAC oligomers form mitochondrial pores to release mtDNA fragments and promote lupus-like disease. Science. 2019;366:1531–6.
Seo W, Gao Y, He Y, Sun J, Xu H, Feng D, et al. ALDH2 deficiency promotes alcohol-associated liver cancer by activating oncogenic pathways via oxidized DNA-enriched extracellular vesicles. J Hepatol. 2019;71:1000–11.
Garcia-Martinez I, Santoro N, Chen Y, Hoque R, Ouyang X, Caprio S, et al. Hepatocyte mitochondrial DNA drives nonalcoholic steatohepatitis by activation of TLR9. J Clin Invest. 2016;126:859–64.
Aswani A, Manson J, Itagaki K, Chiazza F, Collino M, Wupeng WL, et al. Scavenging circulating mitochondrial DNA as a potential therapeutic option for multiple organ dysfunction in trauma hemorrhage. Front Immunol. 2018;9:891.
Bae M, Lee Y, Park YK, Shin DG, Joshi P, Hong SH, et al. Astaxanthin attenuates the increase in mitochondrial respiration during the activation of hepatic stellate cells. J Nutr Biochem. 2019;71:82–89.
Kong D, Chen L, Huang W, Zhang Z, Wang L, Zhang F, et al. Combined therapy with ligustrazine and paeonol mitigates hepatic fibrosis through destroying mitochondrial integrity of stellate cell. Am J Transl Res. 2020;12:1255–66.
Acknowledgements
This work was supported by the Beijing Municipal Science & Technology Commission (Grant No. 7212174 to XL); National Natural Science Foundation of China (Grant No. 82004045 to XL); Beijing Nova Program of Science & Technology (Grant No. Z191100001119088 to XL; Grant No. Z201100006820025 to RL); and the Young Talents Promotion Project of China Association of Traditional Chinese Medicine (Grant No. 2020-QNRC2-01 to XL); Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (Grant No. ZYYCXTD-C-202006 to XL).
Author information
Authors and Affiliations
Contributions
XL and RPL conceived the original idea and supervised the study. XL, YJL, and RPL prepared the manuscript and figures. YJL, MND, QZ, JZW, XYX, YQG, BNM, YJC, Shuo Li, Sheng Lin, and LYZ conducted all the experiments. YJL, MND, JZW, and XYX performed data analysis. All authors have approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Li, Yj., Liu, Rp., Ding, Mn. et al. Tetramethylpyrazine prevents liver fibrotic injury in mice by targeting hepatocyte-derived and mitochondrial DNA-enriched extracellular vesicles. Acta Pharmacol Sin 43, 2026–2041 (2022). https://doi.org/10.1038/s41401-021-00843-w
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-021-00843-w
Keywords
This article is cited by
-
Targeted blockade of extracellular vesicles-mediated profibrotic vicious circle using EVs-based dual-drug delivery system to prevent liver fibrosis
Journal of Nanobiotechnology (2025)
-
Tetramethylpyrazine alleviates hepatic fibrosis induced by experimental mansonic schistosomiasis
Inflammopharmacology (2025)
-
Si-Wu-Tang improves liver fibrosis by restoring liver sinusoidal endothelial cell functionality and reducing communication with hepatic stellate cells
Chinese Medicine (2024)
-
Extracellular vesicles: opening up a new perspective for the diagnosis and treatment of mitochondrial dysfunction
Journal of Nanobiotechnology (2024)
-
Mitochondria-derived vesicles and their potential roles in kidney stone disease
Journal of Translational Medicine (2023)