Abstract
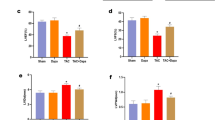
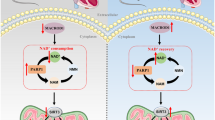
Obesity is an important independent risk factor for cardiovascular diseases, remaining an important health concern worldwide. Evidence shows that saturated fatty acid-induced inflammation in cardiomyocytes contributes to obesity-related cardiomyopathy. Dapagliflozin (Dapa), a selective SGLT2 inhibitor, exerts a favorable preventive activity in heart failure. In this study, we investigated the protective effect of Dapa against cardiomyopathy caused by high fat diet-induced obesity in vitro and in vivo. Cultured rat cardiomyocyte H9c2 cells were pretreated with Dapa (1, 2.5 μM) for 1.5 h, followed by treatment with palmitic acid (PA, 200 μM) for 24 h. We showed that Dapa pretreatment concentration-dependently attenuated PA-induced cell hypertrophy, fibrosis and apoptosis. Transcriptome analysis revealed that inhibition of PA-activated MAPK/AP-1 pathway contributed to the protective effect of Dapa in H9c2 cells, and this was confirmed by anti-p-cJUN fluorescence staining assay. Using surface plasmon resonance analysis we found the direct binding of Dapa with NHE1. Gain and loss of function experiments further demonstrated the role of NHE1 in the protection of Dapa. In vivo experiments were conducted in mice fed a high fat diet for 5 months. The mice were administered Dapa (1 mg·kg−1·d−1, i.g.) in the last 2 months. Dapa administration significantly reduced the body weight and improved the serum lipid profiles. Dapa administration also alleviated HFD-induced cardiac dysfunction and cardiac aberrant remodeling via inhibiting MAPK/AP-1 pathway and ameliorating cardiac inflammation. In conclusion, Dapa exerts a direct protective effect against saturated fatty acid-induced cardiomyocyte injury in addition to the lowering effect on serum lipids. The protective effect results from negative regulating MAPK/AP-1 pathway in a NHE1-dependent way. The current study highlights the potential of clinical use of Dapa in the prevention of obesity-related cardiac dysfunction.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Horwich TB, Fonarow GC, Clark AL. Obesity and the obesity paradox in heart failure. Prog Cardiovasc Dis. 2018;61:151–6.
Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev. 2008;88:389–419.
Tiwari S, Ndisang JF. The role of obesity in cardiomyopathy and nephropathy. Curr Pharmacol Des. 2014;20:1409–17.
Kennedy A, Martinez K, Chuang CC, LaPoint K, McIntosh M. Saturated fatty acid-mediated inflammation and insulin resistance in adipose tissue: mechanisms of action and implications. J Nutr. 2009;139:1–4.
Schaeffler A, Gross P, Buettner R, Bollheimer C, Buechler C, Neumeier M, et al. Fatty acid-induced induction of Toll-like receptor-4/nuclear factor-kappaB pathway in adipocytes links nutritional signalling with innate immunity. Immunology 2009;126:233–45.
Nakamura M, Sadoshima J. Cardiomyopathy in obesity, insulin resistance and diabetes. J Physiol. 2020;598:2977–93.
Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–24.
McMurray JJV, DeMets DL, Inzucchi SE, Køber L, Kosiborod MN, Langkilde AM, et al. A trial to evaluate the effect of the sodium-glucose co-transporter 2 inhibitor dapagliflozin on morbidity and mortality in patients with heart failure and reduced left ventricular ejection fraction (DAPA-HF). Eur J Heart Fail. 2019;21:665–75.
Li N, Zhou H. SGLT2 inhibitors: a novel player in the treatment and prevention of diabetic cardiomyopathy. Drug Des Devel Ther. 2020;14:4775–88.
Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–6.
Wang D, Luo Y, Wang X, Orlicky DJ, Myakala K, Yang P, et al. The sodium-glucose cotransporter 2 inhibitor dapagliflozin prevents renal and liver disease in western diet induced obesity mice. Int J Mol Sci. 2018;19:137.
Xu L, Nagata N, Nagashimada M, Zhuge F, Ni Y, Chen G, et al. SGLT2 inhibition by empagliflozin promotes fat utilization and browning and attenuates inflammation and insulin resistance by polarizing M2 macrophages in diet-induced obese mice. EBioMedicine. 2017;20:137–49.
Lee JY, Lee M, Lee JY, Bae J, Shin E, Lee YH, et al. Ipragliflozin, an SGLT2 Inhibitor, ameliorates high-fat diet-induced metabolic changes by upregulating energy expenditure through activation of the AMPK/SIRT1 pathway. Diabetes Metab J. 2021;45:921–32.
Yokono M, Takasu T, Hayashizaki Y, Mitsuoka K, Kihara R, Muramatsu Y, et al. SGLT2 selective inhibitor ipragliflozin reduces body fat mass by increasing fatty acid oxidation in high-fat diet-induced obese rats. Eur J Pharmacol. 2014;727:66–74.
Uthman L, Baartscheer A, Schumacher CA, Fiolet JWT, Kuschma MC, Hollmann MW, et al. Direct cardiac actions of sodium glucose cotransporter 2 inhibitors target pathogenic mechanisms underlying heart failure in diabetic patients. Front Physiol. 2018;9:1575.
Zhang H, Uthman L, Bakker D, Sari S, Chen S, Hollmann MW, et al. Empagliflozin decreases lactate generation in an nhe-1 dependent fashion and increases α-ketoglutarate synthesis from palmitate in type ii diabetic mouse hearts. Front Cardiovasc Med. 2020;7:592233.
Jiang K, Xu Y, Wang D, Chen F, Tu Z, Qian J, et al. Cardioprotective mechanism of SGLT2 inhibitor against myocardial infarction is through reduction of autosis. Protein Cell. 2021. https://doi.org/10.1007/s13238-020-00809-4.
Bayes-Genis A, Iborra-Egea O, Spitaleri G, Domingo M, Revuelta-López E, Codina P, et al. Decoding empagliflozin’s molecular mechanism of action in heart failure with preserved ejection fraction using artificial intelligence. Sci Rep. 2021;11:12025.
Xie Z, Bailey A, Kuleshov MV, Clarke DJB, Evangelista JE, Jenkins SL, et al. Gene set knowledge discovery with Enrichr. Curr Protoc. 2021;1:e90.
Dong Y, Gao Y, Ilie A, Kim D, Boucher A, Li B, et al. Structure and mechanism of the human NHE1-CHP1 complex. Nat Commun. 2021;12:3474.
Koyani CN, Plastira I, Sourij H, Hallström S, Schmidt A, Rainer PP, et al. Empagliflozin protects heart from inflammation and energy depletion via AMPK activation. Pharmacol Res. 2020;158:104870.
Nicolaides NC, Correa I, Casadevall C, Travali S, Soprano KJ, Calabretta B. The Jun family members, c-Jun and JunD, transactivate the human c-myb promoter via an Ap1-like element. J Biol Chem. 1992;267:19665–72.
Wang Y, Qian Y, Fang Q, Zhong P, Li W, Wang L, et al. Saturated palmitic acid induces myocardial inflammatory injuries through direct binding to TLR4 accessory protein MD2. Nat Commun. 2017;8:13997.
Chung YJ, Park KC, Tokar S, Eykyn TR, Fuller W, Pavlovic D, et al. Off-target effects of SGLT2 blockers: empagliflozin does not inhibit Na+/H+ exchanger-1 or lower [Na+]i in the heart. Cardiovasc Res. 2021;12:2794–806.
Pedersen SF, Darborg BV, Rentsch ML, Rasmussen M. Regulation of mitogen-activated protein kinase pathways by the plasma membrane Na+/H+ exchanger, NHE1. Arch Biochem Biophys. 2007;462:195–201.
Hayashi T, Fukui T, Nakanishi N, Yamamoto S, Tomoyasu M, Osamura A, et al. Dapagliflozin decreases small dense low-density lipoprotein-cholesterol and increases high-density lipoprotein 2-cholesterol in patients with type 2 diabetes: comparison with sitagliptin. Cardiovasc Diabetol. 2017;16:8.
Chen X, Das R, Komorowski R, Beres A, Hessner MJ, Mihara M, et al. Blockade of interleukin-6 signaling augments regulatory T-cell reconstitution and attenuates the severity of graft-versus-host disease. Blood. 2009;114:891–900.
Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev. 2012;92:689–737.
Ebbesson SO, Voruganti VS, Higgins PB, Fabsitz RR, Ebbesson LO, Laston S, et al. Fatty acids linked to cardiovascular mortality are associated with risk factors. Int J Circumpolar Health. 2015;74:28055.
Fuster JJ, Ouchi N, Gokce N, Walsh K. Obesity-induced changes in adipose tissue microenvironment and their impact on cardiovascular disease. Circ Res. 2016;118:1786–807.
Larsen TS, Jansen KM. Impact of obesity-related inflammation on cardiac metabolism and function. J Lipid Atheroscler. 2021;10:8–23.
Makki K, Froguel P, Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm. 2013;2013:139239.
Mouton AJ, Li X, Hall ME, Hall JE. Obesity, hypertension, and cardiac dysfunction: novel roles of immunometabolism in macrophage activation and inflammation. Circ res. 2020;126:789–806.
Prieur X, Roszer T, Ricote M. Lipotoxicity in macrophages: evidence from diseases associated with the metabolic syndrome. Biochim Biophys Acta. 2010;1801:327–37.
He Y, Zhou L, Fan Z, Liu S, Fang W. Palmitic acid, but not high-glucose, induced myocardial apoptosis is alleviated by N‑acetylcysteine due to attenuated mitochondrial-derived ROS accumulation-induced endoplasmic reticulum stress. Cell Death Dis. 2018;9:568.
Mangali S, Bhat A, Udumula MP, Dhar I, Sriram D, Dhar A. Inhibition of protein kinase R protects against palmitic acid-induced inflammation, oxidative stress, and apoptosis through the JNK/NF-kB/NLRP3 pathway in cultured H9c2 cardiomyocytes. J Cell Biochem. 2019;120:3651–63.
Huang S, Rutkowsky JM, Snodgrass RG, Ono-Moore KD, Schneider DA, Newman JW, et al. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J Lipid Res. 2012;53:2002–13.
Jaiswal AK, Makhija S, Stahr N, Sandey M, Suryawanshi A, Saxena A, et al. Dendritic cell-restricted progenitors contribute to obesity-associated airway inflammation via ADAM17-p38 MAPK-dependent pathway. Front Immunol. 2020;11:363.
Wang S, Gu J, Xu Z, Zhang Z, Bai T, Xu J, et al. Zinc rescues obesity-induced cardiac hypertrophy via stimulating metallothionein to suppress oxidative stress-activated BCL10/CARD9/p38 MAPK pathway. J Cell Mol Med. 2017;21:1182–92.
Luo M, Luo P, Zhang Z, Payne K, Watson S, Wu H, et al. Zinc delays the progression of obesity-related glomerulopathy in mice via down-regulating P38 MAPK-mediated inflammation. Obesity (Silver Spring). 2016;24:1244–56.
Solinas G, Becattini B. JNK at the crossroad of obesity, insulin resistance, and cell stress response. Mol Metab. 2017;6:174–84.
Uthman L, Baartscheer A, Bleijlevens B, Schumacher CA, Fiolet JWT, Koeman A, et al. Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na+/H+ exchanger, lowering of cytosolic Na+ and vasodilation. Diabetologia. 2018;61:722–6.
Yeves AM, Ennis IL. Na+/H+ exchanger and cardiac hypertrophy. Hipertens Riesgo Vasc. 2020;37:22–32.
Goss GG, Jiang L, Vandorpe DH, Kieller D, Chernova MN, Robertson M, et al. Role of JNK in hypertonic activation of Cl−-dependent Na+/H+ exchange in Xenopus oocytes. Am J Physiol Cell Physiol. 2001;281:C1978–90.
Pederson SF, Varming C, Christensen ST, Hoffmann EK. Mechanisms of activation of NHE by cell shrinkage and by calyculin A in Ehrlich ascites tumor cells. J Membr Biol. 2002;189:67–81.
Takahashi E, Abe J, Gallis B, Aebersold R, Spring DJ, Krebs EG, et al. p90(RSK) is a serum-stimulated Na+/H+ exchanger isoform-1 kinase. Regulatory phosphorylation of serine 703 of Na+/H+ exchanger isoform-1. J Biol Chem. 1999;274:20206–14.
Khaled AR, Moor AN, Li A, Kim K, Ferris DK, Muegge K, et al. Trophic factor withdrawal: p38 mitogen-activated protein kinase activates NHE1, which induces intracellular alkalinization. Mol Cell Biol. 2001;21:7545–57.
Yamazaki T, Komuro I, Kudoh S, Zou Y, Nagai R, Aikawa R, et al. Role of ion channels and exchangers in mechanical stretch-induced cardiomyocyte hypertrophy. Circ Res. 1998;82:430–7.
Aker S, Snabaitis AK, Konietzka I, Van De Sand A, Böngler K, Avkiran M, et al. Inhibition of the Na+/H+ exchanger attenuates the deterioration of ventricular function during pacing-induced heart failure in rabbits. Cardiovasc Res. 2004;63:273–82.
Javadov S, Baetz D, Rajapurohitam V, Zeidan A, Kirshenbaum LA, Karmazyn M. Anti-hypertrophic effect of Na+/H+ exchanger isoform 1 inhibition is mediated by reduced mitogen-activated protein kinase activation secondary to improved mitochondrial integrity and decreased generation of mitochondrial-derived reactive oxygen species. J Pharmacol Exp Ther. 2006;317:1036–43.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81600341), the Medical Health Science and Technology Project of Zhejiang Province (2021RC091), the Natural Science Foundation of Zhejiang Province (LQ15H020005), Wenzhou Science Technology Bureau Foundation (Y20190616), and Zhejiang Provincial Science and Technology Innovation Program (New Young Talent Program) for College Students (2021R413084).
Author information
Authors and Affiliations
Contributions
PRS and WJH contributed to the literature search and study design. KL, NY and CXY carried out the cellular experiments. KL and WL carried out the analysis for RNA-Sequence and molecular docking. KL and JFQ carried out the experiments on gain and loss of NHE1. KL, NY and DYX carried out the in vivo experiments. KL, CXY and WL contributed to the statistical analysis. KL drafted the article. GL, PRS and WJH revised the article before submission. NY and WWZ carried out the experiments in revision stage. KL, SJY, GL, WJH and PRS revised the article in the revision stage.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Lin, K., Yang, N., Luo, W. et al. Direct cardio-protection of Dapagliflozin against obesity-related cardiomyopathy via NHE1/MAPK signaling. Acta Pharmacol Sin 43, 2624–2635 (2022). https://doi.org/10.1038/s41401-022-00885-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-022-00885-8
Keywords
This article is cited by
-
Heart failure with preserved ejection fraction and obesity: emerging metabolic therapeutic strategies
Diabetology & Metabolic Syndrome (2025)
-
Rhein alleviates hepatic steatosis in NAFLD mice by activating the AMPK/ACC/SREBP1 pathway to enhance lipid metabolism
Molecular Medicine (2025)
-
L-Fucose alleviates inflammation, pyroptosis and mitochondrial injury in obesity-related cardiac injury via TLR4/MyD88/NF-κB pathway
Journal of Translational Medicine (2025)
-
SGLT2 inhibitors: how do they affect the cardiac cells
Molecular and Cellular Biochemistry (2025)
-
Class Effect of SGLT2 Inhibitors Against Doxorubicin-Induced Cardiotoxicity via Regulating Adenosine Kinase Mediated-Cardiac Oxidative Stress
Cardiovascular Drugs and Therapy (2025)