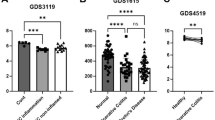
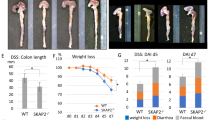
Abstract
Hederacoside C (HSC) has attracted much attention as a novel modulator of inflammation, but its anti-inflammatory mechanism remains elusive. In the present study, we investigated how HSC attenuated intestinal inflammation in vivo and in vitro. HSC injection significantly alleviated TNBS-induced colitis by inhibiting pro-inflammatory cytokine production and colonic epithelial cell apoptosis, and partially restored colonic epithelial cell proliferation. The therapeutic effect of HSC injection was comparable to that of oral administration of mesalazine (200 mg·kg−1·d−1, i.g.). In LPS-stimulated human intestinal epithelial Caco-2 cells, pretreatment with HSC (0.1, 1, 10 μM) significantly inhibited activation of MAPK/NF-κB and its downstream signaling pathways. Pretreatment with HSC prevented LPS-induced TLR4 dimerization and MyD88 recruitment in vitro. Quantitative proteomic analysis revealed that HSC injection regulated 18 proteins in the colon samples, mainly clustered in neutrophil degranulation. Among them, S100A9 involved in the degranulation of neutrophils was one of the most significantly down-regulated proteins. HSC suppressed the expression of S100A9 and its downstream genes including TLR4, MAPK, and NF-κB axes in colon. In Caco-2 cells, recombinant S100A9 protein activated the MAPK/NF-κB signaling pathway and induced inflammation, which were ameliorated by pretreatment with HSC. Notably, HSC attenuated neutrophil recruitment and degranulation as well as S100A9 release in vitro and in vivo. In addition, HSC promoted the expression of tight junction proteins and repaired the epithelial barrier via inhibiting S100A9. Our results verify that HSC ameliorates colitis via restoring impaired intestinal barrier through moderating S100A9/MAPK and neutrophil recruitment inactivation, suggesting that HSC is a promising therapeutic candidate for colitis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Windsor JW, Kaplan GG. Evolving epidemiology of IBD. Curr Gastroenterol Rep. 2019;21:40.
Flynn S, Eisenstein S. Inflammatory bowel disease presentation and diagnosis. Surg Clin North Am. 2019;99:1051–62.
Metzger JC, Kurz E, Von Spee-Mayer C, Kolck G, Bogumil A, Galle PR, et al. Chronic granulomatous disease as a rare differential diagnosis of inflammatory bowel disease. Z Gastroenterol. 2018;56:1507–12.
Zhou M, He J, Shen Y, Zhang C, Wang J, Chen Y. New frontiers in genetics, gut microbiota, and immunity: A rosetta stone for the pathogenesis of inflammatory bowel disease. Biomed Res Int. 2017;2017:8201672.
Ebbo M, Crinier A, Vely F, Vivier E. Innate lymphoid cells: major players in inflammatory diseases. Nat Rev Immunol. 2017;17:665–78.
Pickard JM, Zeng MY, Caruso R, Nunez G. Gut microbiota: role in pathogen colonization, immune responses, and inflammatory disease. Immunol Rev. 2017;279:70–89.
Ramos GP, Papadakis KA. Mechanisms of disease: inflammatory bowel diseases. Mayo Clin Proc. 2019;94:155–65.
Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141–53.
Groschwitz KR, Hogan SP. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol. 2009;124:3–20.
Zihni C, Mills C, Matter K, Balda MS. Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol. 2016;17:564–80.
Blander JM. Death in the intestinal epithelium-basic biology and implications for inflammatory bowel disease. FEBS J. 2016;283:2720–30.
Coskun M. Intestinal epithelium in inflammatory bowel disease. Front Med. 2014;1:24.
Bonovas S, Fiorino G, Allocca M, Lytras T, Nikolopoulos GK, Peyrin-Biroulet L, et al. Biologic therapies and risk of infection and malignancy in patients with inflammatory bowel disease: A systematic review and network meta-analysis. Clin Gastroenterol Hepatol. 2016;14:1385–97.e10.
Xu HC, Wu B, Ma YM, Xu H, Shen ZH, Chen S. Hederacoside-C protects against AGEs-induced ECM degradation in mice chondrocytes. Int Immunopharmacol. 2020;84:106579.
Akhtar M, Shaukat A, Zahoor A, Chen Y, Wang Y, Yang M, et al. Hederacoside-C inhibition of staphylococcus aureus-induced mastitis via TLR2 & TLR4 and their downstream signaling NF-kappaB and MAPKs pathways in vivo and in vitro. Inflammation. 2020;43:579–94.
Kang NX, Zhu YJ, Zhao JP, Zhu WF, Liu YL, Xu QM, et al. Antischistosomal activity of hederacochiside C against schistosoma japonicum harbored in experimentally infected animals. J Asian Nat Prod Res. 2017;19:402–15.
Joh EH, Jeong JJ, Kim DH. Kalopanaxsaponin B inhibits LPS-induced inflammation by inhibiting IRAK1 Kinase. Cell Immunol. 2012;279:103–8.
Requena P, Daddaoua A, Martinez-Plata E, Gonzalez M, Zarzuelo A, Suarez MD, et al. Bovine glycomacropeptide ameliorates experimental rat ileitis by mechanisms involving downregulation of interleukin 17. Br J Pharmacol. 2008;154:825–32.
Jiang H, Yang J, Zhang W, Wang Q, Du Y, Sun Q, et al. Characterisation of hederacoside C metabolites using ultrahigh-performance liquid chromatography quadrupole orbitrap mass spectrometry based on automatic fragment ion search. Phytochem Anal. 2020;31:395–407.
Peeters L, Beirnaert C, Van der Auwera A, Bijttebier S, De Bruyne T, Laukens K, et al. Revelation of the metabolic pathway of hederacoside C using an innovative data analysis strategy for dynamic multiclass biotransformation experiments. J Chromatogr A. 2019;1595:240–7.
Zhang Y, Zha Z, Shen W, Li D, Kang N, Chen Z, et al. Anemoside B4 ameliorates TNBS-induced colitis through S100A9/MAPK/NF-kappaB signaling pathway. Chin Med. 2021;16:11.
Duan Q, Li D, Xiong L, Chang Z, Xu G. SILAC Quantitative proteomics and biochemical analyses reveal a novel molecular mechanism by which ADAM12S promotes the proliferation, migration, and invasion of small cell lung cancer cells through upregulating hexokinase 1. J Proteome Res. 2019;18:2903–14.
Liu P, Bian Y, Liu T, Zhong J, Zhong Y, Zhuang S, et al. Huai hua san alleviates dextran sulphate sodium-induced colitis and modulates colonic microbiota. J Ethnopharmacol. 2020;259:112944.
Hennessy EJ, Parker AE, O’Neill LA. Targeting Toll-like receptors: emerging therapeutics? Nat Rev Drug Discov. 2010;9:293–307.
Zhang X, Wei L, Wang J, Qin Z, Wang J, Lu Y, et al. Suppression colitis and colitis-associated colon cancer by anti-S100a9 antibody in mice. Front Immunol. 2017;8:1774.
Bressenot A, Salleron J, Bastien C, Danese S, Boulagnon-Rombi C, Peyrin-Biroulet L. Comparing histological activity indexes in UC. Gut. 2015;64:1412–8.
Wera O, Lancellotti P, Oury C. The dual role of neutrophils in inflammatory bowel diseases. J Clin Med. 2016;5:118.
Zhao J, Gao W, Cai X, Xu J, Zou D, Li Z, et al. Nanozyme-mediated catalytic nanotherapy for inflammatory bowel disease. Theranostics. 2019;9:2843–55.
Zhou G, Yu L, Fang L, Yang W, Yu T, Miao Y, et al. CD177+ neutrophils as functionally activated neutrophils negatively regulate IBD. Gut. 2018;67:1052–63.
Akhtar M, Shaukat A, Zahoor A, Chen Y, Wang Y, Yang M, et al. Anti-inflammatory effects of Hederacoside-C on staphylococcus aureus induced inflammation via TLRs and their downstream signal pathway in vivo and in vitro. Micro Pathog. 2019;137:103767.
Tsukamoto H, Fukudome K, Takao S, Tsuneyoshi N, Kimoto M. Lipopolysaccharide-binding protein-mediated Toll-like receptor 4 dimerization enables rapid signal transduction against lipopolysaccharide stimulation on membrane-associated CD14-expressing cells. Int Immunol. 2010;22:271–80.
Sands BE. Biomarkers of inflammation in inflammatory bowel disease. Gastroenterology. 2015;149:1275–85.e2.
Xu X, Chen H, Zhu X, Ma Y, Liu Q, Xue Y, et al. S100A9 promotes human lung fibroblast cells activation through receptor for advanced glycation end-product-mediated extracellular-regulated kinase 1/2, mitogen-activated protein-kinase and nuclear factor-kappaB-dependent pathways. Clin Exp Immunol. 2013;173:523–35.
Wang S, Song R, Wang Z, Jing Z, Wang S, Ma J. S100A8/A9 in inflammation. Front Immunol. 2018;9:1298.
Silvin A, Chapuis N, Dunsmore G, Goubet AG, Dubuisson A, Derosa L, et al. Elevated calprotectin and abnormal myeloid cell subsets discriminate severe from mild COVID-19. Cell. 2020;182:1401–18.e18.
Nemeth T, Sperandio M, Mocsai A. Neutrophils as emerging therapeutic targets. Nat Rev Drug Discov. 2020;19:253–75.
Mortaz E, Alipoor SD, Adcock IM, Mumby S, Koenderman L. Update on neutrophil function in severe inflammation. Front Immunol. 2018;9:2171.
Buechler C, Pohl R, Aslanidis C. Pro-resolving molecules-new approaches to treat sepsis? Int J Mol Sci. 2017;18:476.
Zhang LC, Wang Y, Tong LC, Sun S, Liu WY, Zhang S, et al. Berberine alleviates dextran sodium sulfate-induced colitis by improving intestinal barrier function and reducing inflammation and oxidative stress. Exp Ther Med. 2017;13:3374–82.
Bian X, Yang L, Wu W, Lv L, Jiang X, Wang Q, et al. Pediococcus pentosaceus LI05 alleviates DSS-induced colitis by modulating immunological profiles, the gut microbiota, and short-chain fatty acid levels in a mouse model. Micro Biotechnol. 2020;13:1228–44.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 82073912), Suzhou Science and Technology Plan Project (SYS2019032), and a project funded by Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Contributions
YLL and QMX designed the research. ZXZ, YL, KXW, and DL conducted the experiments. ZXZ, YL, YLZ, and YLL wrote the manuscript. GQX, QMX, YL, YLZ, and YLL revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Zha, Zx., Lin, Y., Wang, Kx. et al. Hederacoside C ameliorates colitis via restoring impaired intestinal barrier through moderating S100A9/MAPK and neutrophil recruitment inactivation. Acta Pharmacol Sin 44, 105–119 (2023). https://doi.org/10.1038/s41401-022-00933-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-022-00933-3
Keywords
This article is cited by
-
Hederasaponin C ameliorates chronic obstructive pulmonary disease pathogenesis by targeting TLR4 to inhibit NF-κB/MAPK signaling pathways
Chinese Medicine (2025)
-
Exogenous TSG-6 treatment alleviates DSS-induced colitis in mice by modulating Pou2f3 and promoting tuft cells differentiation
Molecular Medicine (2025)