Abstract
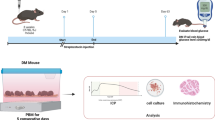
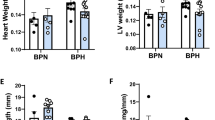
It is of great clinical significance to develop potential novel strategies to prevent diabetic cardiovascular complications. Endothelial progenitor cell (EPC) dysfunction is a key contributor to diabetic vascular complications. In the present study we evaluated whether low-dose nifedipine could rescue impaired EPC-mediated angiogenesis and prevent cardiovascular complications in diabetic mice. Diabetes was induced in mice by five consecutive injections of streptozotocin (STZ, 60 mg·kg−1·d−1, i.p.). Diabetic mice were treated with low-dose nifedipine (1.5 mg·kg−1·d−1, i.g.) for six weeks. Then, circulating EPCs in the peripheral blood were quantified, and bone marrow-derived EPCs (BM-EPCs) were prepared. We showed that administration of low-dose nifedipine significantly increased circulating EPCs, improved BM-EPCs function, promoted angiogenesis, and reduced the cerebral ischemic injury in diabetic mice. Furthermore, we found that low-dose nifedipine significantly increased endothelial nitric oxide synthase (eNOS) expression and intracellular NO levels, and decreased the levels of intracellular O2.− and thrombospondin-1/2 (TSP-1/2, a potent angiogenesis inhibitor) in BM-EPCs of diabetic mice. In cultured BM-EPCs, co-treatment with nifedipine (0.1, 1 μM) dose-dependently protected against high-glucose-induced impairment of migration, and suppressed high-glucose-induced TSP-1 secretion and superoxide overproduction. In mice with middle cerebral artery occlusion, intravenous injection of diabetic BM-EPCs treated with nifedipine displayed a greater ability to promote local angiogenesis and reduce cerebral ischemic injury compared to injection of diabetic BM-EPCs treated with vehicle, and the donor-derived BM-EPCs homed to the recipient ischemic brain. In conclusion, low-dose nifedipine can enhance EPCs’ angiogenic potential and protect against cerebral ischemic injury in diabetic mice. It is implied that chronic treatment with low-dose nifedipine may be a safe and economic manner to prevent ischemic diseases (including stroke) in diabetes.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Xu Y, Wang LM, He J, Bi YF, Li M, Wang TG, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948–59.
Dluhy RG, McMahon GT. Intensive glycemic control in the ACCORD and ADVANCE trials. N Engl J Med. 2008;358:2630–3.
Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59.
ADVANCE Collaborative Group, Patel A, MacMahon S, Chalmers J, Neal B, Billot L, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72.
Phipps MS, Jastreboff AM, Furie K, Kernan WN. The diagnosis and management of cerebrovascular disease in diabetes. Curr Diab Rep. 2012;12:314–23.
Loomans CJM, Koning EJPde, Staal FJT, Rookmaaker MB, Verseyden C, Boer HCde, et al. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53:195–9.
Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–6.
Hill JM, Zalos G, Halcox JPJ, Schenke WH, Waclawiw MA, Quyyumi AA, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600.
Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007.
Fan YF, Shen FX, Frenzel T, Zhu W, Ye JQ, Liu JR, et al. Endothelial progenitor cell transplantation improves long-term stroke outcome in mice. Ann Neurol. 2010;67:488–97.
Sugiura T, Kondo T, Kureishi-Bando Y, Numaguchi Y, Yoshida O, Dohi Y. et al. Nifedipine improves endothelial function: role of endothelial progenitor cells. Hypertension. 2008;52:491–8.
Zauli G, Toffoli B, Iasio MGdi, Celeghini C, Fabris B, Secchiero P. Treatment with recombinant tumor necrosis factor-related apoptosis-inducing ligand alleviates the severity of streptozotocin-induced diabetes. Diabetes. 2010;59:1261–5.
Wang XR, Zhang MW, Chen DD, Zhang Y, Chen AF. AMP-activated protein kinase rescues the angiogenic functions of endothelial progenitor cells via manganese superoxide dismutase induction in type 1 diabetes. Am J Physiol Endocrinol Metab. 2011;300:E1135–45.
Simpkins JW, Rajakumar G, Zhang YQ, Simpkins CE, Greenwald D, Yu CJ, et al. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J Neurosurg. 1997;87:724–30.
Suzuki S, Brown CM, Wise PM. Neuroprotective effects of estrogens following ischemic stroke. Front Neuroendocrinol. 2009;30:201–11.
Ju LN, McFadyen JD, Al-Daher S, Alwis I, Chen YF, Tønnesen LL, et al. Compression force sensing regulates integrin αIIbβ3 adhesive function on diabetic platelets. Nat Commun. 2018;9:1087.
Marrotte EJ, Chen DD, Hakim JS, Chen AF. Manganese superoxide dismutase expression in endothelial progenitor cells accelerates wound healing indiabetic mice. J Clin Invest. 2010;120:4207–19.
Dong XH, Sun X, Jiang GJ, Chen AF, Xie HH. Dietary intake of sugar substitutes aggravates cerebral ischemic injury and impairs endothelial progenitor cells in mice. Stroke. 2015;46:1714–8.
Xie HH, Zhou S, Chen DD, Channon KM, Su DF, Chen AF. GTP cyclohydrolase I/BH4 pathway protects EPCs via suppressing oxidative stress and thrombospondin-1 in salt-sensitive hypertension. Hypertension. 2010;56:1137–44.
Dong XH, Peng C, Zhang YY, Jiang Y, Yang LJ, He JB, et al. Low-dose piperlongumine rescues impaired function of endothelial progenitor cells and reduces cerebral ischemic injury in high-fat diet-fed mice. Front Pharmacol. 2021;12:689880.
Dong XH, Peng C, Zhang YY, Tao YL, Tao X, Zhang C. et al. Chronic exposure to subtherapeutic antibiotics aggravates ischemic stroke outcome in mice. EBioMedicine. 2017;24:116–26.
Tran MD, Neary JT. Purinergic signaling induces thrombospondin-1 expression in astrocytes. Proc Natl Acad Sci USA. 2006;103:9321–6.
Zhu W, Fan YF, Frenzel T, Gasmi M, Bartus RT, Young WL, et al. Insulin growth factor−1 gene transfer enhances neurovascular remodeling and improves long-term stroke outcome in mice. Stroke. 2008;39:1254–61.
Zhang ZG, Zhang L, Jiang Q, Chopp M. Bone marrow-derived endothelial progenitor cells participate in cerebral neovascularization after focal cerebral ischemia in the adult mouse. Circ Res. 2002;90:284–8.
Chen JL, Zhang CL, Jiang H, Li Y, Zhang LJ, Robin A, et al. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25:281–90.
Awal HB, Nandula SR, Domingues CC, Dore FJ, Kundu N, Brichacek B, et al. Linagliptin, when compared to placebo, improves CD34+ve endothelial progenitor cells in type 2 diabetes subjects with chronic kidney disease taking metformin and/or insulin: a randomized controlled trial. Cardiovasc Diabetol. 2020;19:72.
Kito T, Shibata R, Kondo M, Yamamoto T, Suzuki H, Ishii M, et al. Nifedipine ameliorates ischemia-induced revascularization in diet-induced obese mice. Am J Hypertens. 2012;25:401–6.
Miao XN, Siu KL, Cai H. Nifedipine attenuation of abdominal aortic aneurysm in hypertensive and non-hypertensive mice: mechanisms and implications. J Mol Cell Cardiol. 2015;87:152–9.
Thum T, Fraccarollo D, Schultheiss M, Froese S, Galuppo P, Widder JD, et al. Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes. Diabetes. 2007;56:666–74.
Adams GN, LaRusch GA, Stavrou E, Zhou YH, Nieman MT, Jacobs GH, et al. Murine prolylcarboxypeptidase depletion induces vascular dysfunction with hypertension and faster arterial thrombosis. Blood. 2011;117:3929–37.
Sato N, Kaneko M, Tamura M, Kurumatani H. The prostacyclin analog beraprost sodium ameliorates characteristics of metabolic syndrome in obese Zucker (fatty) rats. Diabetes. 2010;59:1092–100.
Bae O, Wang JM, Baek SH, Wang QD, Yuan H, Chen AF. Oxidative stress-mediated thrombospondin-2 upregulation impairs bone marrow-derived angiogenic cell function in diabetes mellitus. Arterioscler Thromb Vasc Biol. 2013;33:1920–7.
Li M, Takenaka H, Asai J, Ibusuki K, Mizukami Y, Maruyama K, et al. Endothelial progenitor thrombospondin-1 mediates diabetes-induced delay in reendothelialization following arterial injury. Circ Res. 2006;98:697–704.
Acknowledgements
This work was supported by grants from the Ministry of Science and Technology of China (2021YFA0804803), the National Natural Science Foundation of China (81870189, 82070266, 81900243, 81930012, 81730013, 92168120 and 81974506), and the Beijing Natural Science Foundation (Z200019).
Author information
Authors and Affiliations
Contributions
HHX conceived and designed the experiments. CP, LJY, YJ, LWXS, JBH and ZWZ performed the experiments. HHX, LT, CZ and AFC analyzed and interpreted the data. XT provided intellectual contribution and useful discussion. HHX and CP wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Peng, C., Yang, Lj., Zhang, C. et al. Low-dose nifedipine rescues impaired endothelial progenitor cell-mediated angiogenesis in diabetic mice. Acta Pharmacol Sin 44, 44–57 (2023). https://doi.org/10.1038/s41401-022-00948-w
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-022-00948-w