Abstract
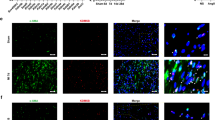
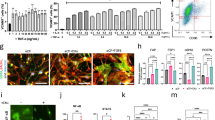
Long-term treatment with adriamycin (ADR) is associated with higher incidences of cumulative cardiotoxicity manifest as heart failure. ADR-induced cardiomyopathy is characterized by extensive fibrosis that is caused by cardiac fibroblast activation. To date, however, no specific treatment is available to alleviate ADR-induced cardiotoxicity. Protein arginine methyltransferase 5 (PRMT5), a major enzyme responsible for methylation of arginine, regulates numerous cellular processes such as cell differentiation. In the present study we investigated the role of PRMT5 in cardiac fibrosis. Mice were administered ADR (3 mg/kg, i.p., every 2 days) for 2 weeks. We showed that aberrant PRMT5 expression was largely co-localized with α-SMA-positive activated cardiac fibroblasts in ADR-injected mice and in ADR-treated cardiac fibroblasts in vitro. PRMT5-overexpression exacerbated, whereas PRMT5 knockdown alleviated ADR-induced cardiac fibrosis in vivo and TGF-β1-induced cardiac fibroblast activation in vitro. We demonstrated that PRMT5-overexpression enhanced methylated-Smad3 levels in vivo and in vitro. Pretreatment with a specific PRMT5 inhibitor EPZ015666 (5 nM) or overexpression of a catalytically inactive mutant of PRMT5, PRMT5(E444Q), reduced PRMT5-induced methylation of Smad3, thus suppressing PRMT5-mediated cardiac fibroblast activation in vitro. Furthermore, ADR activated cardiac fibroblasts was depending on autocrine TGF-β1. Taken together, our results demonstrate that PRMT5 promotes ADR-induced cardiac fibrosis via activating cardiac fibroblasts, suggesting that it may be a potential therapeutic target of ADR-caused cardiotoxicity.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Carvalho FS, Burgeiro A, Garcia R, Moreno AJ, Carvalho RA, Oliveira PJ. Doxorubicin-induced cardiotoxicity: from bioenergetic failure and cell death to cardiomyopathy. Med Res Rev. 2014;34:106–35.
Liu J, Cai Q, Wang W, Lu M, Liu J, Zhou F, et al. Ginsenoside Rh2 pretreatment and withdrawal reactivated the pentose phosphate pathway to ameliorate intracellular redox disturbance and promoted intratumoral penetration of adriamycin. Redox Biol. 2020;32:101452.
Anninga JK, Gelderblom H, Fiocco M, Kroep JR, Taminiau AHM, Hogendoorn PCW, et al. Chemotherapeutic adjuvant treatment for osteosarcoma: where do we stand? Eur J Cancer. 2011;47:2431–45.
Navarro-Hortal MD, Varela-Lopez A, Romero-Marquez JM, Rivas-Garcia L, Speranza L, Battino M, et al. Role of flavonoids against adriamycin toxicity. Food Chem Toxicol. 2020;146:111820.
Lother A, Bergemann S, Kowalski J, Huck M, Gilsbach R, Bode C, et al. Inhibition of the cardiacmyocyte mineralocorticoid receptor ameliorates doxorubicin-induced cardiotoxicity. Cardiovasc Res. 2018;114:282–90.
Zhang Y-W, Shi J, Li Y-J, Wei L. Cardiomyocyte death in doxorubicin-induced cardiotoxicity. Arch Immunol Ther Exp. 2009;57:435–45.
Takemura G, Fujiwara H. Doxorubicin-induccd cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis. 2007;49:330–52.
Frangogiannis NG. Cardiac fibrosis. Cardiovasc Res. 2021;117:1450–88.
Varricchi G, Ameri P, Cadeddu C, Ghigo A, Madonna R, Marone G, et al. Antineoplastic drug-induced cardiotoxicity: a redox perspective. Front Physiol. 2018;9:167.
Medina-Gomez C, Bolanos J, Borbolla-Vazquez J, Munguia-Robledo S, Orozco E, Rodriguez MA. The atypical protein arginine methyltrasferase of Entamoeba histolytica (EhPRMTA) is involved in cell proliferation, heat shock response and in vitro virulence. Exp Parasitol. 2021;222:108077.
Liu M-Y, Hua W-K, Chen C-J, Lin W-J. The MKK-dependent phosphorylation of p38 alpha is augmented by arginine methylation on Arg49/Arg149 during erythroid differentiation. Int J Mol Sci. 2020;21:3546.
Zhu J, Zhang D, Liu X, Yu G, Cai X, Xu C, et al. Zebrafish prmt5 arginine methyltransferase is essential for germ cell development. Development. 2019;146:dev179572.
Stopa N, Krebs JE, Shechter D. The PRMT5 arginine methyltransferase: many roles in development, cancer and beyond. Cell Mol Life Sci. 2015;72:2041–59.
Kim H, Ronai ZeA. PRMT5 function and targeting in cancer. Cell Stress. 2020;4:199–215.
Calabretta S, Vogel G, Yu Z, Choquet K, Darbelli L, Nicholson TB, et al. Loss of PRMT5 promotes PDGFR alpha degradation during oligodendrocyte differentiation and myelination. Dev Cell. 2018;46:426–40.
Kota SK, Roening C, Patel N, Kota SB, Baron R. PRMT5 inhibition promotes osteogenic differentiation of mesenchymal stromal cells and represses basal interferon stimulated gene expression. Bone. 2018;117:37–46.
Liu Z, Ramachandran J, Vokes SA, Gray RS. Regulation of terminal hypertrophic chondrocyte differentiation in Prmt5 mutant mice modeling infantile idiopathic scoliosis. Dis Model Mech. 2019;12:dmm041251.
Kutner RH, Zhang XY, Reiser J. Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors. Nat Protoc. 2009;4:495–505.
Du M, Huang K, Huang D, Yang L, Gao L, Wang X, et al. Renalase is a novel target gene of hypoxia-inducible factor-1 in protection against cardiac ischaemia-reperfusion injury. Cardiovasc Res. 2015;105:182–91.
da Silva AR, Neri EA, Turaca LT, Dariolli R, Fonseca-Alaniz MH, Santos-Miranda A, et al. NOTCH1 is critical for fibroblast-mediated induction of cardiomyocyte specialization into ventricular conduction system-like cells in vitro. Sci Rep. 2020;10:16163.
Dong X, Yang Y, Zhou Y, Bi X, Zhao N, Zhang Z, et al. Glutathione S-transferases P1 protects breast cancer cell from adriamycin-induced cell death through promoting autophagy. Cell Death Differ. 2019;26:2086–99.
Chen X, Tong G, Fan J, Shen Y, Wang N, Gong W, et al. FGF21 promotes migration and differentiation of epidermal cells during wound healing via SIRT1-dependent autophagy. Br J Pharmacol. 2021;179:1102–21.
Guo W, Qiu W, Ao X, Li W, He X, Ao L, et al. Low-concentration DMSO accelerates skin wound healing by Akt/mTOR-mediated cell proliferation and migration in diabetic mice. Br J Pharmacol. 2020;177:3327–41.
Tallquist MD, Molkentin JD. Redefining the identity of cardiac fibroblasts. Nat Rev Cardiol. 2017;14:484–91.
Khalil H, Kanisicak O, Prasad V, Correll RN, Fu X, Schips T, et al. Fibroblast-specific TGF-beta-Smad2/3 signaling underlies cardiac fibrosis. J Clin Invest. 2017;127:3770–83.
Fang L, Zhang L, Wei W, Jin X, Wang P, Tong Y, et al. A methylation-phosphorylation switch determines Sox2 stability and function in ESC maintenance or differentiation. Mol Cell. 2014;55:537–51.
Stempien-Otero A, Kim DH, Davis J. Molecular networks underlying myofibroblast fate and fibrosis. J Mol Cell Cardiol. 2016;97:153–61.
Narikawa M, Umemura M, Tanaka R, Hikichi M, Nagasako A, Fujita T, et al. Doxorubicin induces trans-differentiation and MMP1 expression in cardiac fibroblasts via cell death-independent pathways. PLoS ONE. 2019;14:e0221940.
Pardoux C, Derynck R. JNK regulates expression and autocrine signaling of TGF-beta1. Mol Cell. 2004;15:170–1.
Tian X-Q, Ni X-W, Xu H-L, Zheng L, ZhuGe D-L, Chen B, et al. Prevention of doxorubicin-induced cardiomyopathy using targeted MaFGF mediated by nanoparticles combined with ultrasound-targeted MB destruction. Int J Nanomed. 2017;12:7103–19.
El-Said NT, Mohamed EA, Taha RA. Irbesartan suppresses cardiac toxicity induced by doxorubicin via regulating the p38-MAPK/NF-kappa B and TGF-beta 1 pathways. Naunyn Schmiedebergs Arch Pharmacol. 2019;392:647–58.
Sun Z, Lu W, Lin N, Lin H, Zhang J, Ni T, et al. Dihydromyricetin alleviates doxorubicin-induced cardiotoxicity by inhibiting NLRP3 inflammasome through activation of SIRT1. Biochem Pharmacol. 2020;175:113888.
Xu S, Wang Y, Yu M, Wang D, Liang Y, Chen Y, et al. LongShengZhi capsule inhibits doxorubicin-induced heart failure by antioxidative stress. Biomed Pharmacother. 2020;123:109803.
Sun X, Chen G, Xie Y, Jiang D, Han J, Chen F, et al. Qiliqiangxin improves cardiac function and attenuates cardiac remodelling in doxorubicin-induced heart failure rats. Pharm Biol. 2020;58:417–26.
Wang BQ, Hao JM, Jones SC, Yee MS, Roth JC, Dixon IMC. Decreased Smad 7 expression contributes to cardiac fibrosis in the infarcted rat heart. Am J Physiol Heart Circ Physiol. 2002;282:H1685–96.
Szabo Z, Magga J, Alakoski T, Ulvila J, Piuhola J, Vainio L, et al. Connective tissue growth factor inhibition attenuates left ventricular remodeling and dysfunction in pressure overload-induced heart failure. Hypertension. 2014;63:1235–40.
Zamaraev AV, Kopeina GS, Prokhorova EA, Zhivotovsky B, Lavrik IN. Post-translational modification of caspases: the other side of apoptosis regulation. Trends Cell Biol. 2017;27:322–39.
Deribe YL, Pawson T, Dikic I. Post-translational modifications in signal integration. Nat Struct Mol Biol. 2010;17:666–72.
Guo J, Dai X, Laurent B, Zheng N, Gan W, Zhang J, et al. AKT methylation by SETDB1 promotes AKT kinase activity and oncogenic functions. Nat Cell Biol. 2019;21:226–37.
Wang G, Long J, Gao Y, Zhang W, Han F, Xu C, et al. SETDB1-mediated methylation of Akt promotes its K63-linked ubiquitination and activation leading to tumorigenesis. Nat Cell Biol. 2019;21:214–25.
Li W, Wang HY, Zhao X, Duan H, Cheng B, Liu Y, et al. A methylation-phosphorylation switch determines Plk1 kinase activity and function in DNA damage repair. Sci Adv. 2019;5:eaau7566.
Nicholson TB, Chen T, Richard S. The physiological and pathophysiological role of PRMT1-mediated protein arginine methylation. Pharmacol Res. 2009;60:466–74.
Bedford MT, Clarke SG. Protein arginine methylation in mammals: who, what, and why. Mol Cell. 2009;33:1–13.
Biggar KK, Li SSC. Non-histone protein methylation as a regulator of cellular signalling and function. Nat Rev Mol Cell Biol. 2015;16:5–17.
Wei H, Mundade R, Lange KC, Lu T. Protein arginine methylation of non-histone proteins and its role in diseases. Cell Cycle. 2014;13:32–41.
Hsu JM, Chen CT, Chou CK, Kuo HP, Li LY, Lin CY, et al. Crosstalk between Arg 1175 methylation and Tyr 1173 phosphorylation negatively modulates EGFR-mediated ERK activation. Nat Cell Biol. 2011;13:174–81.
He X, Zhu Y, Lin Y-C, Li M, Du J, Dong H, et al. PRMT1-mediated FLT3 arginine methylation promotes maintenance of FLT3-ITD+ acute myeloid leukemia. Blood. 2019;134:548–60.
Guo X, Waddell DS, Wang W, Wang Z, Liberati NT, Yong S, et al. Ligand-dependent ubiquitination of Smad3 is regulated by casein kinase 1 gamma 2, an inhibitor of TGF-beta signaling. Oncogene. 2008;27:7235–47.
Guo X, Ramirez A, Waddell DS, Li Z, Liu X, Wang XF. Axin and GSK3- control Smad3 protein stability and modulate TGF- signaling. Genes Dev. 2008;22:106–20.
Acknowledgements
This work was financially supported by grants from the National Natural Science Foundation of China (Grant nos: 81973322, 81902706), Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province, the Fundamental Research Funds for the Central Universities (Grant nos: JUSRP221037, JUSRP22007, JUSRP122055), Jiangsu Province “Six Summit Talents” Program (Grant no: YY‐038) and Wuxi Taihu Talent Project.
Author information
Authors and Affiliations
Contributions
LLP and JS designed the study and revised the paper. XLD, SZY, BHY, and HL performed experiments. XLD and XHP analysed the data. XLD wrote the paper. LLP and JS reviewed the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dong, Xl., Yuan, Bh., Yu, Sz. et al. Adriamycin induces cardiac fibrosis in mice via PRMT5-mediated cardiac fibroblast activation. Acta Pharmacol Sin 44, 573–583 (2023). https://doi.org/10.1038/s41401-022-00963-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-022-00963-x