Abstract
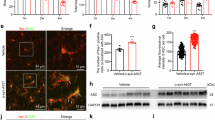
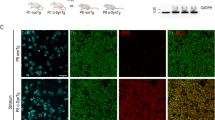
Cell senescence has been implicated in the pathology of Parkinson’s disease (PD). Both abnormal α-synuclein aggregation and iron deposition are suggested to be the triggers, facilitators, and aggravators during the development of PD. In this study, we investigated the involvement of α-synuclein and iron in the process of cell senescence in a mouse model of PD. In order to overexpress α-syn-A53T in the substantia nigra pars compacta (SNpc), human α-syn-A53T was microinjected into both sides of the SNpc in mice. We found that overexpression of α-syn-A53T for one week induced significant pro-inflammatory senescence-associated secretory phenotype (SASP), increased cell senescence-related proteins (β-gal, p16, p21, H2A.X and γ-H2A.X), mitochondrial dysfunction accompanied by dysregulation of iron-related proteins (L-ferritin, H-ferritin, DMT1, IRP1 and IRP2) in the SNpc. In contrast, significant loss of nigral dopaminergic neurons and motor dysfunction were only observed after overexpression of α-syn-A53T for 4 weeks. In PC12 cells stably overexpressing α-syn-A53T, iron overload (ferric ammonium citrate, FAC, 100 μM) not only increased the level of reactive oxygen species (ROS), p16 and p21, but also exacerbated the processes of oxidative stress and cell senescence signalling induced by α-syn-A53T overexpression. Interestingly, reducing the iron level with deferoxamine (DFO) or knockdown of transferrin receptor 1 (TfR1) significantly improved both the phenotypes and dysregulated proteins of cell senescence induced by α-syn-A53T overexpression. All these evidence highlights the toxic interaction between iron and α-synuclein inducing cell senescence, which precedes nigral dopaminergic neuronal loss in PD. Further investigation on cell senescence may yield new therapeutic agents for the prevention or treatment of PD.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Jankovic J, Tan EK. Parkinson’s disease: etiopathogenesis and treatment. J Neurol Neurosurg Psychiatry. 2020;91:795–808.
Guiney SJ, Adlard PA, Bush AI, Finkelstein DI, Ayton S. Ferroptosis and cell death mechanisms in Parkinson’s disease. Neurochem Int. 2017;104:34–48.
Riessland M, Kolisnyk B, Kim TW, Cheng J, Ni J, Pearson JA, et al. Loss of SATB1 induces p21-dependent cellular senescence in post-mitotic dopaminergic neurons. Cell Stem Cell. 2019;25:514–530.e518.
Martinez-Cue C, Rueda N. Cellular senescence in neurodegenerative diseases. Front Cell Neurosci. 2020;14:16.
Miwa S, Kashyap S, Chini E & von Zglinicki T. Mitochondrial dysfunction in cell senescence and aging. J Clin Invest. 2022;132:e158447.
Baker DJ, Petersen RC. Cellular senescence in brain aging and neurodegenerative diseases: evidence and perspectives. J Clin Invest. 2018;128:1208–16.
Schmitz M, Candelise N, Canaslan S, Altmeppen HC, Matschke J, Glatzel M, et al. Alpha-synuclein conformers reveal link to clinical heterogeneity of alpha-synucleinopathies. Transl Neurodegener. 2023;12:12.
Johnson ME, Stecher B, Labrie V, Brundin L, Brundin P. Triggers, facilitators, and aggravators: redefining Parkinson’s disease pathogenesis. Trends Neurosci. 2019;42:4–13.
Wang Z, Luo XG, Gao C. Utility of susceptibility-weighted imaging in Parkinson’s disease and atypical Parkinsonian disorders. Transl Neurodegener. 2016;5:17.
Chen B, Wen X, Jiang H, Wang J, Song N, Xie J. Interactions between iron and alpha-synuclein pathology in Parkinson’s disease. Free Radic Biol Med. 2019;141:253–60.
Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC. Alpha-synuclein is degraded by both autophagy and the proteasome. J Biol Chem. 2003;278:25009–13.
Chen LL, Wang YB, Song JX, Deng WK, Lu JH, Ma LL, et al. Phosphoproteome-based kinase activity profiling reveals the critical role of MAP2K2 and PLK1 in neuronal autophagy. Autophagy. 2017;13:1969–80.
Tie L, Xiao H, Wu DL, Yang Y, Wang P. A brief guide to good practices in pharmacological experiments: Western blotting. Acta Pharmacol Sin. 2021;42:1015–7.
Oliveras-Salva M, Van der Perren A, Casadei N, Stroobants S, Nuber S, D’Hooge R, et al. rAAV2/7 vector-mediated overexpression of alpha-synuclein in mouse substantia nigra induces protein aggregation and progressive dose-dependent neurodegeneration. Mol Neurodegener. 2013;8:44.
Ip CW, Klaus LC, Karikari AA, Visanji NP, Brotchie JM, Lang AE, et al. AAV1/2-induced overexpression of A53T-alpha-synuclein in the substantia nigra results in degeneration of the nigrostriatal system with Lewy-like pathology and motor impairment: a new mouse model for Parkinson’s disease. Acta Neuropathol Commun. 2017;5:11.
Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: an expanding universe. Cell. 2023;186:243–78.
Friesner JD, Liu B, Culligan K, Britt AB. Ionizing radiation-dependent gamma-H2AX focus formation requires ataxia telangiectasia mutated and ataxia telangiectasia mutated and Rad3-related. Mol Biol Cell. 2005;16:2566–76.
Kinner A, Wu W, Staudt C, Iliakis G. Gamma-H2AX in recognition and signaling of DNA double-strand breaks in the context of chromatin. Nucleic Acids Res. 2008;36:5678–94.
Fan T, Kang H, Wu D, Zhu X, Huang L, Wu J, et al. Arabidopsis gamma-H2A.X-INTERACTING PROTEIN participates in DNA damage response and safeguards chromatin stability. Nat Commun. 2022;13:7942.
Castellani RJ, Siedlak SL, Perry G, Smith MA. Sequestration of iron by Lewy bodies in Parkinson’s disease. Acta Neuropathol. 2000;100:111–4.
Subramaniam SR, Chesselet MF. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Prog Neurobiol. 2013;106–107:17–32.
Chen LL, Huang YJ, Cui JT, Song N, Xie J. Iron dysregulation in parkinson’s disease: focused on the autophagy-lysosome pathway. ACS Chem Neurosci. 2019;10:863–71.
Chinta SJ, Woods G, Demaria M, Rane A, Zou Y, McQuade A, et al. Cellular senescence is induced by the environmental neurotoxin paraquat and contributes to neuropathology linked to Parkinson’s disease. Cell Rep. 2018;22:930–40.
Cohen J, Torres C. Astrocyte senescence: evidence and significance. Aging Cell. 2019;18:e12937.
Cancio-Bello A, Saez-Atienzar S. SATB1 is a dopaminergic neuron-specific regulator of cellular senescence. Mov Disord. 2020;35:235.
Kondo M, Tanaka Y, Kuwabara T, Naito T, Kohwi-Shigematsu T, Watanabe A. SATB1 plays a critical role in establishment of immune tolerance. J Immunol. 2016;196:563–72.
Chang D, Nalls MA, Hallgrimsdottir IB, Hunkapiller J, van der Brug M, Cai F, et al. A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci. Nat Genet. 2017;49:1511–6.
Brichta L, Shin W, Jackson-Lewis V, Blesa J, Yap EL, Walker Z, et al. Identification of neurodegenerative factors using translatome-regulatory network analysis. Nat Neurosci. 2015;18:1325–33.
Huang Y, Zhang L, Song NN, Hu ZL, Chen JY, Ding YQ. Distribution of Satb1 in the central nervous system of adult mice. Neurosci Res. 2011;71:12–21.
Hu X, Mao C, Hu Z, Zhang Z, Zhang S, Yang Z, et al. Association analysis of 15 GWAS-linked loci with Parkinson’s disease in Chinese Han population. Neurosci Lett. 2020;725:134867.
Verma DK, Seo BA, Ghosh A, Ma SX, Hernandez-Quijada K, Andersen JK et al. Alpha-synuclein preformed fibrils induce cellular senescence in Parkinson’s disease models. Cells. 2021;10:1694.
Moreau C, Duce JA, Rascol O, Devedjian JC, Berg D, Dexter D, et al. Iron as a therapeutic target for Parkinson’s disease. Mov Disord. 2018;33:568–74.
Chen L, Li C, Xie J. Axonal iron transport might contribute to iron deposition in Parkinson’s disease. Neurosci Bull. 2021;37:275–7.
Chen L, Xie J. Commentary: The impact of iron deposition on the fear circuit of the brain in patients with Parkinson’s disease and anxiety. Front Aging Neurosci. 2023;15:1223421.
Li Y, Yang C, Wang S, Yang D, Zhang Y, Xu L, et al. Copper and iron ions accelerate the prion-like propagation of alpha-synuclein: a vicious cycle in Parkinson’s disease. Int J Biol Macromol. 2020;163:562–73.
Xiao Y, Chen X, Huang S, Li G, Mo M, Zhang L, et al. Iron promotes alpha-synuclein aggregation and transmission by inhibiting TFEB-mediated autophagosome-lysosome fusion. J Neurochem. 2018;145:34–50.
Davies P, Moualla D, Brown DR. Alpha-synuclein is a cellular ferrireductase. PLoS One. 2011;6:e15814.
Brown DR. alpha-Synuclein as a ferrireductase. Biochem Soc Trans. 2013;41:1513–7.
McDowall JS, Ntai I, Honeychurch KC, Hart JP, Colin P, Schneider BL, et al. Alpha-synuclein ferrireductase activity is detectible in vivo, is altered in Parkinson’s disease and increases the neurotoxicity of DOPAL. Mol Cell Neurosci. 2017;85:1–11.
Baksi S, Tripathi AK, Singh N. Alpha-synuclein modulates retinal iron homeostasis by facilitating the uptake of transferrin-bound iron: Implications for visual manifestations of Parkinson’s disease. Free Radic Biol Med. 2016;97:292–306.
Ortega R, Carmona A, Roudeau S, Perrin L, Ducic T, Carboni E, et al. Alpha-synuclein over-expression induces increased iron accumulation and redistribution in iron-exposed neurons. Mol Neurobiol. 2016;53:1925–34.
Guo JJ, Yue F, Song DY, Bousset L, Liang X, Tang J, et al. Intranasal administration of alpha-synuclein preformed fibrils triggers microglial iron deposition in the substantia nigra of Macaca fascicularis. Cell Death Dis. 2021;12:81.
Bartels T, De Schepper S, Hong S. Microglia modulate neurodegeneration in Alzheimer’s and Parkinson’s diseases. Science. 2020;370:66–9.
Prinz M, Jung S, Priller J. Microglia biology: one century of evolving concepts. Cell. 2019;179:292–311.
Liu CY, Wang X, Liu C, Zhang HL. Pharmacological targeting of microglial activation: new therapeutic approach. Front Cell Neurosci. 2019;13:514–32.
Chen L, Huang Y, Yu X, Lu J, Jia W, Song J, et al. Corynoxine protects dopaminergic neurons through inducing autophagy and diminishing neuroinflammation in rotenone-induced animal models of parkinson’s disease. Front Pharmacol. 2021;12:642900.
Cserep C, Posfai B, Lenart N, Fekete R, Laszlo ZI, Lele Z, et al. Microglia monitor and protect neuronal function through specialized somatic purinergic junctions. Science. 2020;367:528–37.
Wang R, Ren H, Kaznacheyeva E, Lu X, Wang G. Association of glial activation and alpha-synuclein pathology in Parkinson’s disease. Neurosci Bull. 2023;39:479–90.
Choi I, Zhang Y, Seegobin SP, Pruvost M, Wang Q, Purtell K, et al. Microglia clear neuron-released alpha-synuclein via selective autophagy and prevent neurodegeneration. Nat Commun. 2020;11:1386.
Song N, Chen L, Xie J. Alpha-synuclein handling by microglia: activating, combating, and worsening. Neurosci Bull. 2021;37:751–3.
George S, Rey NL, Tyson T, Esquibel C, Meyerdirk L, Schulz E, et al. Microglia affect alpha-synuclein cell-to-cell transfer in a mouse model of Parkinson’s disease. Mol Neurodegener. 2019;14:34.
Xia Y, Zhang G, Han C, Ma K, Guo X, Wan F, et al. Microglia as modulators of exosomal alpha-synuclein transmission. Cell Death Dis. 2019;10:174.
Guo M, Wang J, Zhao Y, Feng Y, Han S, Dong Q, et al. Microglial exosomes facilitate alpha-synuclein transmission in Parkinson’s disease. Brain. 2020;143:1476–97.
Chen X, Hu Y, Cao Z, Liu Q, Cheng Y. Cerebrospinal fluid inflammatory cytokine aberrations in Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis: a systematic review and meta-analysis. Front Immunol. 2018;9:2122.
Krashia P, Cordella A, Nobili A, La Barbera L, Federici M, Leuti A, et al. Blunting neuroinflammation with resolvin D1 prevents early pathology in a rat model of Parkinson’s disease. Nat Commun. 2019;10:3945.
Herdy JR, Traxler L, Agarwal RK, Karbacher L, Schlachetzki JCM, Boehnke L, et al. Increased post-mitotic senescence in aged human neurons is a pathological feature of Alzheimer’s disease. Cell Stem Cell. 2022;29:1637–52.
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (32170984), Natura Science Foundation of Shandong Province (ZR2020YQ23, ZR2023QH110), and Qingdao Postdoctoral Research Project (QDBSH20220202204).
Author information
Authors and Affiliations
Contributions
QQS, XHJ, XZM, CL, and LL were involved in experimental research and data analysis. WTJ, and LQ involved in methodology. JXX and LLC were involved in conceptualization, methodology, writing, and supervision. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shen, Qq., Jv, Xh., Ma, Xz. et al. Cell senescence induced by toxic interaction between α-synuclein and iron precedes nigral dopaminergic neuron loss in a mouse model of Parkinson’s disease. Acta Pharmacol Sin 45, 268–281 (2024). https://doi.org/10.1038/s41401-023-01153-z
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-023-01153-z
Keywords
This article is cited by
-
NLRP3 facilitates α-synuclein-induced dopaminergic neuronal senescence in a mouse model of Parkinson’s disease through SATB1/DNA damage/p21 signaling pathway
Acta Pharmacologica Sinica (2026)
-
Immunosenescence in aging and neurodegenerative diseases: evidence, key hallmarks, and therapeutic implications
Translational Neurodegeneration (2025)
-
The interplay of iron, oxidative stress, and α-synuclein in Parkinson’s disease progression
Molecular Medicine (2025)
-
Investigating the ageing-Parkinson’s disease nexus: standardisation of in vitro models and techniques by the PD-AGE network
npj Parkinson's Disease (2025)
-
Homeostasis and metabolism of iron and other metal ions in neurodegenerative diseases
Signal Transduction and Targeted Therapy (2025)