Abstract
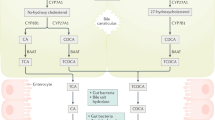
Excessive dietary calories lead to systemic metabolic disorders, disturb hepatic lipid metabolism, and aggravate nonalcoholic steatohepatitis (NASH). Bile acids (BAs) play key roles in regulating nutrition absorption and systemic energy homeostasis. Resmetirom is a selective thyroid hormone receptor β (THRβ) agonist and the first approved drug for NASH treatment. It is well known that the THRβ activation could promote intrahepatic lipid catabolism and improve mitochondrial function, however, its effects on intestinal lipid absorption and BA compositions remain unknown. In the present study, the choline-deficient, L-amino acid defined, high-fat diet (CDAHFD) and high-fat diet plus CCl4 (HFD+CCl4)-induced NASH mice were used to evaluate the effects of resmetirom on lipid and BA composition. We showed that resmetirom administration (10 mg·kg−1·d−1, i.g.) significantly altered hepatic lipid composition, especially reduced the C18:2 fatty acyl chain-containing triglyceride (TG) and phosphatidylcholine (PC) in the two NASH mouse models, suggesting that THRβ activation inhibited intestinal lipid absorption since C18:2 fatty acid could be obtained only from diet. Targeted analysis of BAs showed that resmetirom treatment markedly reduced the hepatic and intestinal 12-OH to non-12-OH BAs ratio by suppressing cytochrome P450 8B1 (CYP8B1) expression in both NASH mouse models. The direct inhibition by resmetirom on intestinal lipid absorption was further verified by the BODIPY gavage and the oral fat tolerance test. In addition, disturbance of the altered BA profiles by exogenous cholic acid (CA) supplementation abolished the inhibitory effects of resmetirom on intestinal lipid absorption in both normal and CDAHFD-fed mice, suggesting that resmetirom inhibited intestinal lipid absorption by reducing 12-OH BAs content. In conclusion, we discovered a novel mechanism of THRβ agonists on NASH treatment by inhibiting intestinal lipid absorption through remodeling BAs composition, which highlights the multiple regulation of THRβ activation on lipid metabolism and extends the current knowledge on the action mechanisms of THRβ agonists in NASH treatment.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77:1335–47.
Xu X, Poulsen KL, Wu L, Liu S, Miyata T, Song Q, et al. Targeted therapeutics and novel signaling pathways in non-alcohol-associated fatty liver/steatohepatitis (NAFL/NASH). Signal Transduct Target Ther. 2022;7:287. https://doi.org/10.1038/s41392-022-01119-3.
Hodson L, Gunn PJ. The regulation of hepatic fatty acid synthesis and partitioning: the effect of nutritional state. Nat Rev Endocrinol. 2019;15:689–700.
Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184:2537–64.
Abenavoli L, Boccuto L, Federico A, Dallio M, Loguercio C, Di Renzo L, et al. Diet and non-alcoholic fatty liver disease: the mediterranean way. Int J Environ Res Public Health. 2019;16:3011. https://doi.org/10.3390/ijerph16173011.
Huby T, Gautier EL. Immune cell-mediated features of non-alcoholic steatohepatitis. Nat Rev Immunol. 2022;22:429–43.
Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94:355–82.
Ritter MJ, Amano I, Hollenberg AN. Thyroid hormone signaling and the liver. Hepatology. 2020;72:742–52.
Kelly MJ, Pietranico-Cole S, Larigan JD, Haynes NE, Reynolds CH, Scott N, et al. Discovery of 2-[3,5-dichloro-4-(5-isopropyl-6-oxo-1,6-dihydropyridazin-3-yloxy)phenyl]-3,5-dioxo-2,3,4,5-tetrahydro[1,2,4]triazine-6-carbonitrile (MGL-3196), a highly selective thyroid hormone receptor β agonist in clinical trials for the treatment of dyslipidemia. J Med Chem. 2014;57:3912–23.
Harrison SA, Taub R, Neff GW, Lucas KJ, Labriola D, Moussa SE, et al. Resmetirom for nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled phase 3 trial. Nat Med. 2023;29:2919–28.
Sinha RA, Singh BK, Yen PM. Direct effects of thyroid hormones on hepatic lipid metabolism. Nat Rev Endocrinol. 2018;14:259–69.
Sinha RA, Singh BK, Zhou J, Wu Y, Farah BL, Ohba K, et al. Thyroid hormone induction of mitochondrial activity is coupled to mitophagy via ROS-AMPK-ULK1 signaling. Autophagy. 2015;11:1341–57.
Sinha RA, Bruinstroop E, Singh BK, Yen PM. Nonalcoholic fatty liver disease and hypercholesterolemia: roles of thyroid hormones, metabolites, and agonists. Thyroid. 2019;29:1173–91.
Li T, Chiang JY. Regulation of bile acid and cholesterol metabolism by PPARs. PPAR Res. 2009;2009:501739. https://doi.org/10.1155/2009/501739.
Chiang JY. Bile acid metabolism and signaling. Compr Physiol. 2013;3:1191–212.
Li-Hawkins J, Gåfvels M, Olin M, Lund EG, Andersson U, Schuster G, et al. Cholic acid mediates negative feedback regulation of bile acid synthesis in mice. J Clin Invest. 2002;110:1191–200.
Chiang JYL, Ferrell JM. Bile acid metabolism in liver pathobiology. Gene Expr. 2018;18:71–87.
Jia W, Wei M, Rajani C, Zheng X. Targeting the alternative bile acid synthetic pathway for metabolic diseases. Protein Cell. 2021;12:411–25.
Lin JZ, Martagon AJ, Hsueh WA, Baxter JD, Gustafsson JA, Webb P, et al. Thyroid hormone receptor agonists reduce serum cholesterol independent of the LDL receptor. Endocrinology. 2012;153:6136–44.
Bonde Y, Breuer O, Lutjohann D, Sjoberg S, Angelin B, Rudling M. Thyroid hormone reduces PCSK9 and stimulates bile acid synthesis in humans. J Lipid Res. 2014;55:2408–15.
Johansson L, Rudling M, Scanlan TS, Lundåsen T, Webb P, Baxter J, et al. Selective thyroid receptor modulation by GC-1 reduces serum lipids and stimulates steps of reverse cholesterol transport in euthyroid mice. Proc Natl Acad Sci USA. 2005;102:10297–302.
Berkenstam A, Kristensen J, Mellström K, Carlsson B, Malm J, Rehnmark S, et al. The thyroid hormone mimetic compound KB2115 lowers plasma LDL cholesterol and stimulates bile acid synthesis without cardiac effects in humans. Proc Natl Acad Sci USA. 2008;105:663–7.
Astapova I, Ramadoss P, Costa-e-Sousa RH, Ye F, Holtz KA, Li Y, et al. Hepatic nuclear corepressor 1 regulates cholesterol absorption through a TRbeta1-governed pathway. J Clin Invest. 2014;124:1976–86.
Yan Y, Niu Z, Sun C, Li P, Shen S, Liu S, et al. Hepatic thyroid hormone signalling modulates glucose homeostasis through the regulation of GLP-1 production via bile acid-mediated FXR antagonism. Nat Commun. 2022;13:6408. https://doi.org/10.1038/s41467-022-34258-w.
Brunt EM, Kleiner DE, Wilson LA, Belt P, Neuschwander-Tetri BA. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53:810–20.
Liang W, Menke AL, Driessen A, Koek GH, Lindeman JH, Stoop R, et al. Establishment of a general NAFLD scoring system for rodent models and comparison to human liver pathology. PLoS One. 2014;9:e115922. https://doi.org/10.1371/journal.pone.0115922.
Sarafian MH, Lewis MR, Pechlivanis A, Ralphs S, Mcphail MJW, Patel VC, et al. Bile acid profiling and quantification in biofluids using ultra-performance liquid chromatography tandem mass spectrometry. Anal Chem. 2015;87:9662–70.
Cajka T, Smilowitz JT, Fiehn O. Validating quantitative untargeted lipidomics across nine liquid chromatographyhigh-resolution mass spectrometry platforms. Anal Chem. 2017;89:12360–8.
Jin HR, Wang J, Wang ZJ, Xi MJ, Xia BH, Deng K, et al. Lipid metabolic reprogramming in tumor microenvironment: from mechanisms to therapeutics. J Hematol Oncol. 2023;16:103. https://doi.org/10.1186/s13045-023-01498-2.
Clifford BL, Sedgeman LR, Williams KJ, Morand P, Cheng A, Jarrett KE, et al. FXR activation protects against NAFLD via bile-acid-dependent reductions in lipid absorption. Cell Metab. 2021;33:1671–84.e4.
Malcicka M, Visser B, Ellers J. An evolutionary perspective on linoleic acid synthesis in animals. Evol Biol. 2018;45:15–26.
Chiang JYL, Ferrell JM. Bile acids as metabolic regulators and nutrient sensors. Annu Rev Nutr. 2019;39:175–200.
Chiang JY. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955–66.
Li T, Chiang JY. Nuclear receptors in bile acid metabolism. Drug Metab Rev. 2013;45:145–55.
Dawson PA, Lan T, Rao A. Bile acid transporters. J Lipid Res. 2009;50:2340–57.
Zhong S, Chèvre R, Castaño Mayan D, Corlianò M, Cochran BJ, Sem KP, et al. Haploinsufficiency of CYP8B1 associates with increased insulin sensitivity in humans. J Clin Invest. 2022;132. https://doi.org/10.1172/JCI152961.
Zhang M, Chiang JY. Transcriptional regulation of the human sterol 12alpha-hydroxylase gene (CYP8B1): roles of heaptocyte nuclear factor 4alpha in mediating bile acid repression. J Biol Chem. 2001;276:41690–9.
Gallage S, Avila JEB, Ramadori P, Focaccia E, Rahbari M, Ali A, et al. A researcher’s guide to preclinical mouse NASH models. Nat Metab. 2022;4:1632–49.
Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397:2212–24.
Vvedenskaya O, Rose TD, Knittelfelder O, Palladini A, Wodke JAH, Schuhmann K, et al. Nonalcoholic fatty liver disease stratification by liver lipidomics. J Lipid Res. 2021;62:100104. https://doi.org/10.1016/j.jlr.2021.100104.
Bartz R, Li WH, Venables B, Zehmer JK, Roth MR, Welti R, et al. Lipidomics reveals that adiposomes store ether lipids and mediate phospholipid traffic. J Lipid Res. 2007;48:837–47.
Tauchi-Sato K, Ozeki S, Houjou T, Taguchi R, Fujimoto T. The surface of lipid droplets is a phospholipid monolayer with a unique Fatty Acid composition. J Biol Chem. 2002;277:44507–12.
Perino A, Schoonjans K. Metabolic messengers: bile acids. Nat Metab. 2022;4:416–23.
Chiang JYL, Ferrell JM. Bile acid receptors FXR and TGR5 signaling in fatty liver diseases and therapy. Am J Physiol Gastrointest Liver Physiol. 2020;318:G554–g73.
Pavlovic N, Golocorbin-Kon S, Ethanic M, Stanimirov B, Al-Salami H, Stankov K, et al. Bile acids and their derivatives as potential modifiers of drug release and pharmacokinetic profiles. Front Pharmacol. 2018;9:1283. https://doi.org/10.3389/fphar.2018.01283.
Li R, Palmiotti A, de Vries HD, Hovingh MV, Koehorst M, Mulder NL, et al. Low production of 12alpha-hydroxylated bile acids prevents hepatic steatosis in Cyp2c70(-/-) mice by reducing fat absorption. J Lipid Res. 2021;62:100134. https://doi.org/10.1016/j.jlr.2021.100134.
Kaur A, Patankar JV, de Haan W, Ruddle P, Wijesekara N, Groen AK, et al. Loss of Cyp8b1 improves glucose homeostasis by increasing GLP-1. Diabetes. 2015;64:1168–79.
Bing H, Li YL. The role of bile acid metabolism in the occurrence and development of NAFLD. Front Mol Biosci. 2022;9:1089359. https://doi.org/10.3389/fmolb.2022.1089359.
Jiao N, Baker SS, Chapa-Rodriguez A, Liu W, Nugent CA, Tsompana M, et al. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut. 2018;67:1881–91.
Mouzaki M, Wang AY, Bandsma R, Comelli EM, Arendt BM, Zhang L, et al. Bile acids and dysbiosis in non-alcoholic fatty liver disease. PLoS One. 2016;11:e0151829. https://doi.org/10.1371/journal.pone.0151829.
Sang C, Wang X, Zhou K, Sun T, Bian H, Gao X, et al. Bile Acid Profiles Are distinct among patients with different etiologies of chronic liver disease. J Proteome Res. 2021;20:2340–51.
Puri P, Daita K, Joyce A, Mirshahi F, Santhekadur PK, Cazanave S, et al. The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids. Hepatology. 2018;67:534–48.
Iwasaki W, Yoshida R, Liu H, Hori S, Otsubo Y, Tanaka Y, et al. The ratio of 12alpha to non-12-hydroxylated bile acids reflects hepatic triacylglycerol accumulation in high-fat diet-fed C57BL/6J mice. Sci Rep. 2022;12:16707. https://doi.org/10.1038/s41598-022-20838-9.
Sanyal AJ, Ling L, Beuers U, DePaoli AM, Lieu HD, Harrison SA, et al. Potent suppression of hydrophobic bile acids by aldafermin, an FGF19 analogue, across metabolic and cholestatic liver diseases. JHEP Rep. 2021;3:100255. https://doi.org/10.1016/j.jhepr.2021.100255.
Hori S, Abe T, Lee DG, Fukiya S, Yokota A, Aso N, et al. Association between 12alpha-hydroxylated bile acids and hepatic steatosis in rats fed a high-fat diet. J Nutr Biochem. 2020;83:108412. https://doi.org/10.1016/j.jnutbio.2020.108412.
Lee JY, Shimizu H, Hagio M, Fukiya S, Watanabe M, Tanaka Y, et al. 12α-Hydroxylated bile acid induces hepatic steatosis with dysbiosis in rats. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865:158811. https://doi.org/10.1016/j.bbalip.2020.158811.
de Aguiar Vallim TQ, Tarling EJ, Edwards PA. Pleiotropic roles of bile acids in metabolism. Cell Metab. 2013;17:657–69.
Chiang JYL, Ferrell JM. Up to date on cholesterol 7 alpha-hydroxylase (CYP7A1) in bile acid synthesis. Liver Res. 2020;4:47–63.
Donepudi AC, Ferrell JM, Boehme S, Choi HS, Chiang JYL. Deficiency of cholesterol 7alpha-hydroxylase in bile acid synthesis exacerbates alcohol-induced liver injury in mice. Hepatol Commun. 2018;2:99–112.
Grevengoed TJ, Trammell SA, Svenningsen JS, Makarov MV, Nielsen TS, Jacobsen JCB, et al. An abundant biliary metabolite derived from dietary omega-3 polyunsaturated fatty acids regulates triglycerides. J Clin Invest. 2021;131. https://doi.org/10.1172/JCI143861.
Higuchi S, Ahmad TR, Argueta DA, Perez PA, Zhao C, Schwartz GJ, et al. Bile acid composition regulates GPR119-dependent intestinal lipid sensing and food intake regulation in mice. Gut. 2020;69:1620–8.
Wang B, Rong X, Duerr MA, Hermanson DJ, Hedde PN, Wong JS, et al. Intestinal phospholipid remodeling is required for dietary-lipid uptake and survival on a high-fat diet. Cell Metab. 2016;23:492–504.
Wit M, Trujillo-Viera J, Strohmeyer A, Klingenspor M, Hankir M, Sumara G. When fat meets the gut-focus on intestinal lipid handling in metabolic health and disease. EMBO Mol Med. 2022;14:e14742. https://doi.org/10.15252/emmm.202114742.
Kowalik MA, Columbano A, Perra A. Thyroid hormones, thyromimetics and their metabolites in the treatment of liver disease. Front Endocrinol. 2018;9:382. https://doi.org/10.3389/fendo.2018.00382.
Goswami R, Tandon RK, Dudha A, Kochupillai N. Prevalence and significance of steatorrhea in patients with active Graves’ disease. Am J Gastroenterol. 1998;93:1122–5.
Misra GC, Bose SL, Samal AK. Malabsorption in thyroid dysfunctions. J Indian Med Assoc. 1991;89:195–7.
Acknowledgements
This project was supported by Shanghai Municipal Science and Technology Major Project and the Foundation of Shanghai Science and Technology Committee (Grant No. 21DZ2291100).
Author information
Authors and Affiliations
Contributions
YL, JL, and KS designed the research. KS, NLZ, SLH, HQ, YPG, and LQ performed the research. KS and NLZ analyzed and interpreted the data. YL, JL, KS, and NLZ wrote the paper. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, K., Zhu, Nl., Huang, Sl. et al. A new mechanism of thyroid hormone receptor β agonists ameliorating nonalcoholic steatohepatitis by inhibiting intestinal lipid absorption via remodeling bile acid profiles. Acta Pharmacol Sin 45, 2134–2148 (2024). https://doi.org/10.1038/s41401-024-01303-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-024-01303-x
Keywords
This article is cited by
-
Repurposing levothyroxine for managing metabolic dysfunction-associated steatotic liver disease
Endocrine (2025)
-
Revisiting the relationship between thyroid function and metabolic dysfunction-associated steatotic liver disease in the era of resmetirom
Journal of Gastroenterology (2025)
-
Resmetirom, the first FDA-approved drug for MASH: From drug discovery and action mechanisms to clinical trials
Archives of Pharmacal Research (2025)