Abstract
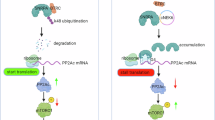
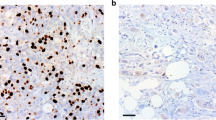
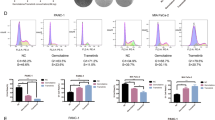
Pancreatic ductal adenocarcinoma (PDAC) is distinguished by its aggressive malignancy, limited treatment avenues and a tendency towards chemotherapy resistance, underscoring the critical need for advanced research to uncover new therapeutic approaches. Stress granules (SGs) that is implicated in cellular self-protection mechanism, along with its associated family molecules have shown pro-cancer effects and are closely related to tumor chemotherapy resistance. In this study we investigated the relationship between Ras GTPase-activating protein-binding proteins 2 (G3BP2), a core component of SGs, and the malignancy of PDAC as well as its resistance to the chemotherapy drug gemcitabine. Analyzing TCGA dataset revealed that the expression of G3BP1 and G3BP2 was significantly upregulated in PDAC compared with adjacent normal pancreatic tissues, and the high expression of G3BP2 rather than G3BP1 was significantly associated with poorer overall survival (OS) in PDAC patients. We demonstrated that knockdown of G3BP2 inhibited the proliferation and invasion of PANC‐1 and CFPAC-1 cells in vitro and in vivo. By analyzing the differentially expressed genes in G3BP2 knockdown and overexpressed PANC‐1 cells, we identified DKC1 that was associated with RNA stability and regulation as the target of G3BP2. We demonstrated that G3BP2 bound to PDIA3 mRNA and recruited them into SGs, increasing the stability of PDIA3 mRNA and attenuating its translation efficiency, thereby promoting DKC1 expression. Furthermore, DKC1 could bind to hENT mRNA and inhibited its expression, which enhanced gemcitabine resistance of PDAC. Therefore, we propose a novel mechanism wherein G3BP2 facilitates PDAC’s resistance to chemotherapy by modulating PDIA3-DKC1-hENT in a SGs-dependent way, suggesting G3BP2 SGs a protentional therapeutic target for the treatment in PDAC.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Fabbri L, Chakraborty A, Robert C, Vagner S. The plasticity of mRNA translation during cancer progression and therapy resistance. Nat Rev Cancer. 2021;21:558–77.
Labrie M, Brugge JS, Mills GB, Zervantonakis IK. Therapy resistance: opportunities created by adaptive responses to targeted therapies in cancer. Nat Rev Cancer. 2022;22:323–39.
De Santis MC, Gozzelino L, Margaria JP, Costamagna A, Ratto E, Gulluni F, et al. Lysosomal lipid switch sensitises to nutrient deprivation and mTOR targeting in pancreatic cancer. Gut. 2023;72:360–71.
Halbrook CJ, Thurston G, Boyer S, Anaraki C, Jimenez JA, McCarthy A, et al. Differential integrated stress response and asparagine production drive symbiosis and therapy resistance of pancreatic adenocarcinoma cells. Nat Cancer. 2022;3:1386–403.
Jain V, Amaravadi RK. Pumping iron: ferritinophagy promotes survival and therapy resistance in pancreatic cancer. Cancer Discov. 2022;12:2023–5.
Park W, Chawla A, O’Reilly EM. Pancreatic cancer: a review. JAMA. 2021;326:851–62.
Lavalee M, Curdy N, Laurent C, Fournie JJ, Franchini DM. Cancer cell adaptability: turning ribonucleoprotein granules into targets. Trends Cancer. 2021;7:902–15.
Kedersha N, Ivanov P, Anderson P. Stress granules and cell signaling: more than just a passing phase? Trends Biochem Sci. 2013;38:494–506.
Xing F, Qin Y, Xu J, Wang W, Zhang B. Stress granules dynamics and promising functions in pancreatic cancer. Biochim Biophys Acta Rev Cancer. 2023;1878:188885.
Wang X, Chen T, Li C, Li W, Zhou X, Li Y, et al. CircRNA-CREIT inhibits stress granule assembly and overcomes doxorubicin resistance in TNBC by destabilizing PKR. J Hematol Oncol. 2022;15:122.
Grabocka E, Bar-Sagi D. Mutant KRAs enhances tumor cell fitness by upregulating stress granules. Cell. 2016;167:1803–13. e1812
Mukhopadhyay S, Goswami D, Adiseshaiah PP, Burgan W, Yi M, Guerin TM, et al. Undermining glutaminolysis bolsters chemotherapy while NRF2 promotes chemoresistance in KRAS-driven pancreatic cancers. Cancer Res. 2020;80:1630–43.
Fonteneau G, Redding A, Hoag-Lee H, Sim ES, Heinrich S, Gaida MM, et al. Stress granules determine the development of obesity-associated pancreatic cancer. Cancer Discov. 2022;12:1984–2005.
Yang P, Mathieu C, Kolaitis RM, Zhang P, Messing J, Yurtsever U, et al. G3BP1 is a tunable switch that triggers phase separation to assemble stress granules. Cell. 2020;181:325–45. e328
Jin G, Zhang Z, Wan J, Wu X, Liu X, Zhang W. G3BP2: Structure and function. Pharmacol Res. 2022;186:106548.
Sanders DW, Kedersha N, Lee DSW, Strom AR, Drake V, Riback JA, et al. Competing protein-RNA interaction networks control multiphase intracellular organization. Cell. 2020;181:306–24. e328
Ratnadiwakara M, Anko ML. mRNA stability assay using transcription inhibition by actinomycin D in mouse pluripotent stem cells. Bio Protoc. 2018;8:e3072.
Icard P, Fournel L, Wu Z, Alifano M, Lincet H. Interconnection between metabolism and cell cycle in cancer. Trends Biochem Sci. 2019;44:490–501.
Sidibe H, Dubinski A, Vande Velde C. The multi-functional RNA-binding protein G3BP1 and its potential implication in neurodegenerative disease. J Neurochem. 2021;157:944–62.
Jones RJ, Baladandayuthapani V, Neelapu S, Fayad LE, Romaguera JE, Wang M, et al. HDM-2 inhibition suppresses expression of ribonucleotide reductase subunit M2, and synergistically enhances gemcitabine-induced cytotoxicity in mantle cell lymphoma. Blood. 2011;118:4140–9.
Leitner S, Sweeney K, Oberg D, Davies D, Miranda E, Lemoine NR, et al. Oncolytic adenoviral mutants with E1B19K gene deletions enhance gemcitabine-induced apoptosis in pancreatic carcinoma cells and anti-tumor efficacy in vivo. Clin Cancer Res. 2009;15:1730–40.
Ko E, Kim JS, Ju S, Seo HW, Chang Y, Kang JA, et al. Oxidatively modified protein-disulfide isomerase-associated 3 promotes dyskerin pseudouridine synthase 1-mediated malignancy and survival of hepatocellular carcinoma cells. Hepatology. 2018;68:1851–64.
Raffenne J, Nicolle R, Puleo F, Le Corre D, Boyez C, Marechal R, et al. hENT1 testing in pancreatic ductal adenocarcinoma: are we ready? a multimodal evaluation of hENT1 status. Cancers (Basel). 2019;11:1808.
Gupta N, Badeaux M, Liu Y, Naxerova K, Sgroi D, Munn LL, et al. Stress granule-associated protein G3BP2 regulates breast tumor initiation. Proc Natl Acad Sci USA. 2017;114:1033–8.
Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395:2008–20.
Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47–52.
Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73–85.
Qian Y, Gong Y, Fan Z, Luo G, Huang Q, Deng S, et al. Molecular alterations and targeted therapy in pancreatic ductal adenocarcinoma. J Hematol Oncol. 2020;13:130.
Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, et al. Pancreatic adenocarcinoma, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:439–57.
Grasso C, Jansen G, Giovannetti E. Drug resistance in pancreatic cancer: impact of altered energy metabolism. Crit Rev Oncol Hematol. 2017;114:139–52.
Kan G, Wang Z, Sheng C, Chen G, Yao C, Mao Y, et al. Dual inhibition of DKC1 and MEK1/2 synergistically restrains the growth of colorectal cancer cells. Adv Sci (Weinh). 2021;8:2004344.
Prentzell MT, Rehbein U, Cadena Sandoval M, De Meulemeester AS, Baumeister R, Brohee L, et al. G3BPs tether the TSC complex to lysosomes and suppress mTORC1 signaling. Cell. 2021;184:655–74. e627
Wippich F, Bodenmiller B, Trajkovska MG, Wanka S, Aebersold R, Pelkmans L. Dual specificity kinase DYRK3 couples stress granule condensation/dissolution to mTORC1 signaling. Cell. 2013;152:791–805.
Zhao Z, Qing Y, Dong L, Han L, Wu D, Li Y, et al. QKI shuttles internal m7G-modified transcripts into stress granules and modulates mRNA metabolism. Cell. 2023;186:3208–26. e3227
Protter DSW, Parker R. Principles and properties of stress granules. Trends Cell Biol. 2016;26:668–79.
Yang W, Zhang M, Li J, Qu S, Zhou F, Liu M, et al. YTHDF1 mitigates acute kidney injury via safeguarding m6A-methylated mRNAs in stress granules of renal tubules. Redox Biol. 2023;67:102921.
Pederiva C, Trevisan DM, Peirasmaki D, Chen S, Savage SA, Larsson O, et al. Control of protein synthesis through mRNA pseudouridylation by dyskerin. Sci Adv. 2023;9:eadg1805.
Kaehler C, Isensee J, Hucho T, Lehrach H, Krobitsch S. 5-Fluorouracil affects assembly of stress granules based on RNA incorporation. Nucleic Acids Res. 2014;42:6436–47.
Diep CH, Munoz RM, Choudhary A, Von Hoff DD, Han H. Synergistic effect between erlotinib and MEK inhibitors in KRAS wild-type human pancreatic cancer cells. Clin Cancer Res. 2011;17:2744–56.
Acknowledgements
This study was jointly supported by the National Natural Science Foundation of China (82173178, 82173281, U21A20374, and 82072698), Shanghai Municipal Science and Technology Major Project (21JC1401500), Scientific Innovation Project of Shanghai Education Committee (2019-01-07-00-07-E00057), Natural Science Foundation of Shanghai Municipal Science and Technology Committee (21ZR1415200) and Natural Science Foundation of Shanghai (23ZR1479300).
Author information
Authors and Affiliations
Contributions
BZ, YQ, XJY, JX and WW designed research; FLX and BRL performed research; FLX, YJF, CL and JL contributed new analytical tools and reagents; FLX, BRL and YJF analyzed data; FLX wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xing, Fl., Li, Br., Fang, Yj. et al. G3BP2 promotes tumor progression and gemcitabine resistance in PDAC via regulating PDIA3-DKC1-hENT in a stress granules-dependent manner. Acta Pharmacol Sin 46, 474–488 (2025). https://doi.org/10.1038/s41401-024-01387-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-024-01387-5
Keywords
This article is cited by
-
Targeting biomolecular condensates: beyond dissolution
BMC Biology (2026)