Abstract
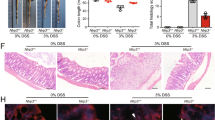
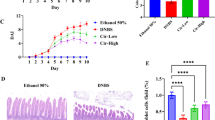
Inflammatory bowel diseases (IBDs) are chronic inflammatory conditions primarily affecting the gastrointestinal tract. Previous studies established the role of the NF-κB signaling pathway in the development of IBDs, suggesting that anti-inflammatory therapies might offer a viable treatment strategy. Tanshinone IIA and salviadione, both derived from Salviae Miltiorrhizae Radix et Rhizoma, possess anti-inflammatory and anti-oxidative activities. A series of new compounds were synthesized by hybridizing salviadione with tanshinone. Among these compounds, 15a showed beneficial effects in LPS-induced acute lung injury and diabetes-induced renal injury mouse models. The current study explored the therapeutic efficacy of 15a using both acute and chronic colitis models and elucidated the underlying mechanisms. DSS-induced colitis models were established in mice, where acute colitis was treated with compound 15a (5 or 10 mg·kg−1·d−1) for 8 days, while chronic colitis mice received compound 15a (5 or 10 mg·kg−1·d−1, i.g.) during 2.5% DSS administration. The 15a treatment significantly alleviated DSS-induced pathological and inflammatory damages in both acute and chronic colitis mouse models. In mouse intestinal epithelial cell line MODE-K, pretreatment with compound 15a (5 or 10 μM) significantly suppressed LPS + L18-MDP-induced inflammatory responses. The receptor-interacting serine/threonine kinase 2 (RIPK2) was identified as a direct binding target of compound 15a using microarrays and recombinant human proteins. Moreover, 15a could directly bind to and inhibit the phosphorylation of RIPK2, leading to the suppression of the NF-κB and MAPK signaling pathways. Furthermore, LEU153 and VAL32 were identified within the KD domain of RIPK2 as critical amino residues for the binding of 15a. Briefly, the current findings demonstrate that compound 15a holds promise as a therapeutic agent for managing acute and chronic colitis.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–29.
Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–17.
Qiu P, Ishimoto T, Fu L, Zhang J, Zhang Z, Liu Y. The gut microbiota in inflammatory bowel disease. Front Cell Infect Microbiol. 2022;12:733992.
Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–8.
Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology. 2017;152:313–21.e2.
Saez A, Herrero-Fernandez B, Gomez-Bris R, Sanchez-Martinez H, Gonzalez-Granado JM. Pathophysiology of inflammatory bowel disease: innate immune system. Int J Mol Sci. 2023;24:1526.
Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–34.
Xiao YT, Yan WH, Cao Y, Yan JK, Cai W. Neutralization of IL-6 and TNF-alpha ameliorates intestinal permeability in DSS-induced colitis. Cytokine. 2016;83:189–92.
Aardoom MA, Veereman G, de Ridder L. A Review on the use of anti-TNF in children and adolescents with inflammatory bowel disease. Int J Mol Sci. 2019;20:2529.
Verstockt B, Ferrante M, Vermeire S, Van Assche G. New treatment options for inflammatory bowel diseases. J Gastroenterol. 2018;53:585–90.
Ashall L, Horton CA, Nelson DE, Paszek P, Harper CV, Sillitoe K, et al. Pulsatile stimulation determines timing and specificity of NF-kappaB-dependent transcription. Science. 2009;324:242–6.
Mukherjee T, Kumar N, Chawla M, Philpott DJ, Basak S. The NF-kappaB signaling system in the immunopathogenesis of inflammatory bowel disease. Sci Signal. 2024;17:eadh1641.
Taniguchi K, Karin M. NF-kappaB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18:309–24.
Strober W, Murray PJ, Kitani A, Watanabe T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol. 2006;6:9–20.
Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–50.
Pham AT, Ghilardi AF, Sun L. Recent advances in the development of RIPK2 modulators for the treatment of inflammatory diseases. Front Pharmacol. 2023;14:1127722.
Hofmann SR, Girschick L, Stein R, Schulze F. Immune modulating effects of receptor interacting protein 2 (RIP2) in autoinflammation and immunity. Clin Immunol. 2021;223:108648.
Jun JC, Cominelli F, Abbott DW. RIP2 activity in inflammatory disease and implications for novel therapeutics. J Leukoc Biol. 2013;94:927–32.
Wang X, Morris-Natschke SL, Lee KH. New developments in the chemistry and biology of the bioactive constituents of Tanshen. Med Res Rev. 2007;27:133–48.
Xu M, Dong MQ, Cao FL, Liu ML, Wang YX, Dong HY, et al. Tanshinone IIA reduces lethality and acute lung injury in LPS-treated mice by inhibition of PLA2 activity. Eur J Pharmacol. 2009;607:194–200.
Guo R, Li L, Su J, Li S, Duncan SE, Liu Z, et al. Pharmacological activity and mechanism of tanshinone IIA in related diseases. Drug Des Devel Ther. 2020;14:4735–48.
Hao H, Wang G, Cui N, Li J, Xie L, Ding Z. Identification of a novel intestinal first pass metabolic pathway: NQO1 mediated quinone reduction and subsequent glucuronidation. Curr Drug Metab. 2007;8:137–49.
Don MJ, Shen CC, Lin YL, Syu WJ, Ding YH, Sun CM. Nitrogen-containing compounds from Salvia miltiorrhiza. J Nat Prod. 2005;68:1066–70.
Ding C, Chen H, Liang B, Jiao M, Liang G, Zhang A. Biomimetic synthesis of the natural product salviadione and its hybrids: discovery of tissue-specific anti-inflammatory agents for acute lung injury. Chem Sci. 2019;10:4667–72.
Li L, Ding C, Zou C, Xiong Z, Zhu W, Qian J, et al. A novel salviadione derivative, compound 15a, attenuates diabetes-induced renal injury by inhibiting NF-kappaB-mediated inflammatory responses. Toxicol Appl Pharmacol. 2020;409:115322.
Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015;12:205–17.
Qu C, Yuan ZW, Yu XT, Huang YF, Yang GH, Chen JN, et al. Patchouli alcohol ameliorates dextran sodium sulfate-induced experimental colitis and suppresses tryptophan catabolism. Pharm Res. 2017;121:70–82.
Nielsen OH, Munck LK. Drug insight: aminosalicylates for the treatment of IBD. Nat Clin Pr Gastroenterol Hepatol. 2007;4:160–70.
Katsandegwaza B, Horsnell W, Smith K. Inflammatory bowel disease: a review of pre-clinical murine models of human disease. Int J Mol Sci. 2022;23:9344.
Honjo H, Watanabe T, Kamata K, Minaga K, Kudo M. RIPK2 as a new therapeutic target in inflammatory bowel diseases. Front Pharmacol. 2021;12:650403.
Larochelle J, Tishko RJ, Yang C, Ge Y, Phan LT, Gunraj RE, et al. Receptor-interacting protein kinase 2 (RIPK2) profoundly contributes to post-stroke neuroinflammation and behavioral deficits with microglia as unique perpetrators. J Neuroinflammation. 2023;20:221.
Barnich N, Aguirre JE, Reinecker HC, Xavier R, Podolsky DK. Membrane recruitment of NOD2 in intestinal epithelial cells is essential for nuclear factor-kappaB activation in muramyl dipeptide recognition. J Cell Biol. 2005;170:21–6.
Bazzoni G, Martinez-Estrada OM, Orsenigo F, Cordenonsi M, Citi S, Dejana E. Interaction of junctional adhesion molecule with the tight junction components ZO-1, cingulin, and occludin. J Biol Chem. 2000;275:20520–6.
Liu S, Kang W, Mao X, Ge L, Du H, Li J, et al. Melatonin mitigates aflatoxin B1-induced liver injury via modulation of gut microbiota/intestinal FXR/liver TLR4 signaling axis in mice. J Pineal Res. 2022;73:e12812.
Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014;20:91–9.
He S, Wang X. RIP kinases as modulators of inflammation and immunity. Nat Immunol. 2018;19:912–22.
Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–51.
Caruso R, Warner N, Inohara N, Nunez G. NOD1 and NOD2: signaling, host defense, and inflammatory disease. Immunity. 2014;41:898–908.
Garcia-Carbonell R, Yao SJ, Das S, Guma M. Dysregulation of intestinal epithelial cell RIPK pathways promotes chronic inflammation in the IBD gut. Front Immunol. 2019;10:1094.
Yu SJ, Liu Y, Deng Y, Zhu XY, Zhan N, Dong WG. CARD3 deficiency protects against colitis through reduced epithelial cell apoptosis. Inflamm Bowel Dis. 2015;21:862–9.
Cooney R, Baker J, Brain O, Danis B, Pichulik T, Allan P, et al. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat Med. 2010;16:90–7.
Larabi A, Barnich N, Nguyen HTT. New insights into the interplay between autophagy, gut microbiota and inflammatory responses in IBD. Autophagy. 2020;16:38–51.
Kim TW, Shin JS, Chung KS, Lee YG, Baek NI, Lee KT. Anti-inflammatory mechanisms of koreanaside A, a lignan isolated from the flower of Forsythia koreana, against LPS-induced macrophage activation and DSS-induced colitis mice: the crucial role of AP-1, NF-kappaB, and JAK/STAT signaling. Cells. 2019;8:1163.
Zhang FX, Kirschning CJ, Mancinelli R, Xu XP, Jin Y, Faure E, et al. Bacterial lipopolysaccharide activates nuclear factor-kappaB through interleukin-1 signaling mediators in cultured human dermal endothelial cells and mononuclear phagocytes. J Biol Chem. 1999;274:7611–4.
Nakase H, Sato N, Mizuno N, Ikawa Y. The influence of cytokines on the complex pathology of ulcerative colitis. Autoimmun Rev. 2022;21:103017.
Lai Y, Wang X, Sun X, Wu S, Chen X, Yang C, et al. Discovery of a novel RIPK2 inhibitor for the treatment of inflammatory bowel disease. Biochem Pharmacol. 2023;214:115647.
Hollenbach E, Neumann M, Vieth M, Roessner A, Malfertheiner P, Naumann M. Inhibition of p38 MAP kinase- and RICK/NF-kappaB-signaling suppresses inflammatory bowel disease. FASEB J. 2004;18:1550–2.
Fiil BK, Gyrd-Hansen M. Met1-linked ubiquitination in immune signalling. FEBS J. 2014;281:4337–50.
Gong Q, Long Z, Zhong FL, Teo DET, Jin Y, Yin Z, et al. Structural basis of RIP2 activation and signaling. Nat Commun. 2018;9:4993.
Hasegawa M, Fujimoto Y, Lucas PC, Nakano H, Fukase K, Nunez G, et al. A critical role of RICK/RIP2 polyubiquitination in Nod-induced NF-kappaB activation. EMBO J. 2008;27:373–83.
Heim VJ, Dagley LF, Stafford CA, Hansen FM, Clayer E, Bankovacki A, et al. A regulatory region on RIPK2 is required for XIAP binding and NOD signaling activity. EMBO Rep. 2020;21:e50400.
Francescone R, Hou V, Grivennikov SI. Cytokines, IBD, and colitis-associated cancer. Inflamm Bowel Dis. 2015;21:409–18.
Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329–42.
Acknowledgements
This study was supported by the Key Scientific Research Project of Wenzhou City (ZY2021021 to YW) and the National Natural Science Foundation of China (82361138563 to YW).
Author information
Authors and Affiliations
Contributions
YW, XHW, GL and CHH contributed to the literature search and study design. YW, XHW and CHH participated in the drafting of the article. CHH, YC, TYJ, ZW, BJ, JL, CYD, AZ, WYT, LXZ, LYX and FMN carried out the experiments. YW, XHW, GL and CHH revised the manuscript. YW, XHW, GL and CHH contributed to data collection and analysis.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, Ch., Chen, Y., Jin, Ty. et al. A derivative of tanshinone IIA and salviadione, 15a, inhibits inflammation and alleviates DSS-induced colitis in mice by direct binding and inhibition of RIPK2. Acta Pharmacol Sin 46, 672–686 (2025). https://doi.org/10.1038/s41401-024-01399-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-024-01399-1