Abstract
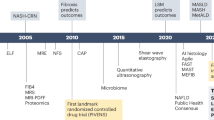
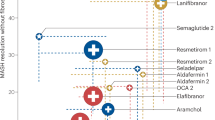
Metabolic dysfunction-associated steatotic liver disease (MASLD) covers a broad spectrum of profile from simple fatty liver, evolving to metabolic dysfunction-associated steatohepatitis (MASH), to hepatic fibrosis, further progressing to cirrhosis and hepatocellular carcinoma (HCC). MASLD has become a prevalent disease with 25% in average over the world. MASH is an active stage, and requires pharmacological intervention when there is necroptotic damage with fibrotic progression. Although there is an increased understanding of MASH pathogenesis and newly approved resmetirom, given its complexity and heterogeneous pathophysiology, there is a strong necessity to develop more drug candidates with better therapeutic efficacy and well-tolerated safety profile. With an increased list of pharmaceutical candidates in the pipeline, it is anticipated to witness successful approval of more potential candidates in this fast-evolving field, thereby offering different categories of medications for selective patient populations. In this review, we update the advances in MASH pharmacotherapeutics that have completed phase II or III clinical trials with potential application in clinical practice during the latest 2 years, focusing on effectiveness and safety issues. The overview of fast-evolving status of pharmacotherapeutic candidates for MASH treatment confers deep insights into the key issues, such as molecular targets, endpoint selection and validation, clinical trial design and execution, interaction with drug administration authority, real-world data feedback and further adjustment in clinical application.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;78:1966–86.
EASL-EASD-EASO. Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J Hepatol. 2024; https://doi.org/10.1016/j.jhep.2024.04.031.
Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:851–61.
Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med. 2017;377:2063–72.
Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397–411.
Zhai M, Liu Z, Long J, Zhou Q, Yang L, Zhou Q, et al. The incidence trends of liver cirrhosis caused by nonalcoholic steatohepatitis via the GBD study 2017. Sci Rep. 2021;11:5195.
Myers S, Neyroud-Caspar I, Spahr L, Gkouvatsos K, Fournier E, Giostra E, et al. NAFLD and MAFLD as emerging causes of HCC: A populational study. JHEP Rep. 2021;3:100231.
Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology. 2014;60:2099–108.
Zhou F, Zhou J, Wang W, Zhang XJ, Ji YX, Zhang P, et al. Unexpected rapid increase in the burden of NAFLD in China from 2008 to 2018: a systematic review and meta-analysis. Hepatology. 2019;70:1119–33.
Man S, Deng Y, Ma Y, Fu J, Bao H, Yu C, et al. Prevalence of liver steatosis and fibrosis in the general population and various high-risk populations: a nationwide study with 5.7 million adults in China. Gastroenterology. 2023;165:1025–40.
Liu H, Qi J, Yang J, Liu F, Li X, Yin P, et al. Burden of liver complications related to non-alcoholic fatty liver disease in China from 2005 to 2019: observations from the global burden of disease study, 2019. Diabetes Obes Metab. 2023;25:43–52.
Brunt EM, Wong VWS, Nobili V, Day CP, Sookoian S, Maher JJ, et al. Nonalcoholic fatty liver disease. Nat Rev Dis Prim. 2015;1:15080.
Noureddin M, Muthiah MD, Sanyal AJ. Drug discovery and treatment paradigms in nonalcoholic steatohepatitis. Endocrinol Diabetes Metab. 2020;3:e00105.
Harrison SA, Bedossa P, Guy CD, Schattenberg JM, Loomba R, Taub R, et al. A phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis. N Engl J Med. 2024;390:497–509.
Kingwell K. NASH field celebrates ‘hurrah moment’ with a first FDA drug approval for the liver disease. Nat Rev Drug Discov. 2024;23:235–7.
Yang YY, Xie L, Zhang NP, Zhou D, Liu TT, Wu J. Updates on novel pharmacotherapeutics for the treatment of nonalcoholic steatohepatitis. Acta Pharmacol Sin. 2022;43:1180–90.
Sinha RA, Bruinstroop E, Singh BK, Yen PM. Nonalcoholic fatty liver disease and hypercholesterolemia: roles of thyroid hormones, metabolites, and agonists. Thyroid. 2019;29:1173–91.
Mendoza A, Tang C, Choi J, Acuna M, Logan M, Martin AG, et al. Thyroid hormone signaling promotes hepatic lipogenesis through the transcription factor ChREBP. Sci Signal. 2021;14:eabh3839.
Taub R, Chiang E, Chabot-Blanchet M, Kelly MJ, Reeves RA, Guertin MC, et al. Lipid lowering in healthy volunteers treated with multiple doses of MGL-3196, a liver-targeted thyroid hormone receptor-β agonist. Atherosclerosis. 2013;230:373–80.
Kelly MJ, Pietranico-Cole S, Larigan JD, Haynes NE, Reynolds CH, Scott N. et al.Discovery of 2-[3,5-dichloro-4-(5-isopropyl-6-oxo-1,6-dihydropyridazin-3-yloxy)phenyl]-3,5-dioxo-2,3,4,5-tetrahydro[1,2,4]triazine-6-carbonitrile (MGL-3196), a highly selective thyroid hormone receptor β agonist in clinical trials for the treatment of dyslipidemia.J Med Chem. 2014;57:3912–23.
Harrison SA, Bashir MR, Guy CD, Zhou R, Moylan CA, Frias JP, et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2019;394:2012–24.
Harrison SA, Taub R, Neff GW, Lucas KJ, Labriola D, Moussa SE, et al. Resmetirom for nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled phase 3 trial. Nat Med. 2023;29:2919–28.
Harrison SA, Taub RA, Ren Y-Y, Chng ELK, Tai D. Artificial intelligence to measure fibrosis change on liver biopsy in MAESTRO-MASH: a phase 3 serial liver biopsy study in 966 patients with MASH treated with resmetirom or placebo. Hepatology. 2023;78:S143–5.
Edfalk S, Steneberg P, Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes. 2008;57:2280–7.
Holst JJ, Toft-Nielsen MB, Orskov C, Nauck M, Willms B. On the effects of glucagon-like peptide-1 on blood glucose regulation in normal and diabetic subjects. Ann N Y Acad Sci. 1996;805:729–36.
Shah M, Vella A. Effects of GLP-1 on appetite and weight. Rev Endocr Metab Disord. 2014;15:181–7.
Qin W, Ying W, Hamaker B, Zhang G. Slow digestion-oriented dietary strategy to sustain the secretion of GLP-1 for improved glucose homeostasis. Compr Rev Food Sci Food Saf. 2021;20:5173–96.
Newsome PN, Ambery P. Incretins (GLP-1 receptor agonists and dual/triple agonists) and the liver. J Hepatol. 2023;79:1557–65.
Sorli C, Harashima SI, Tsoukas GM, Unger J, Karsbøl JD, Hansen T, et al. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017;5:251–60.
Ahrén B, Masmiquel L, Kumar H, Sargin M, Karsbøl JD, Jacobsen SH, et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017;5:341–54.
Ahmann AJ, Capehorn M, Charpentier G, Dotta F, Henkel E, Lingvay I, et al. Efficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56-week, open-label, randomized clinical trial. Diabetes Care. 2018;41:258–66.
Aroda VR, Bain SC, Cariou B, Piletič M, Rose L, Axelsen M, et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5:355–66.
Rodbard HW, Lingvay I, Reed J, de la Rosa R, Rose L, Sugimoto D, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab. 2018;103:2291–301.
Jódar E, Michelsen M, Polonsky W, Réa R, Sandberg A, Vilsbøll T, et al. Semaglutide improves health-related quality of life versus placebo when added to standard of care in patients with type 2 diabetes at high cardiovascular risk (SUSTAIN 6). Diabetes Obes Metab. 2020;22:1339–47.
Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384:989–1002.
Davies M, Færch L, Jeppesen OK, Pakseresht A, Pedersen SD, Perreault L, et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397:971–84.
Wadden TA, Bailey TS, Billings LK, Davies M, Frias JP, Koroleva A, et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA. 2021;325:1403–13.
Rubino D, Abrahamsson N, Davies M, Hesse D, Greenway FL, Jensen C, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: the STEP 4 randomized clinical trial. JAMA. 2021;325:1414–25.
Wester A, Shang Y, Toresson Grip E, Matthews AA, Hagstrom H. Glucagon-like peptide-1 receptor agonists and risk of major adverse liver outcomes in patients with chronic liver disease and type 2 diabetes. Gut. 2024;73:835–43.
Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384:1113–24.
Ratziu V, Patel AS, Krarup NM, Varma S, Noureddin M, Sanyal A. Digital image quantification of the antifibrotic effect of semaglutide and the impact of liver fat in nonalcoholic steatohepatitis. Hepatology. 2023;78:S148–50.
Loomba R, Abdelmalek MF, Armstrong MJ, Jara M, Kjær MS, Krarup N, et al. Semaglutide 2·4 mg once weekly in patients with non-alcoholic steatohepatitis-related cirrhosis: a randomised, placebo-controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2023;8:511–22.
Romero-Gomez M, Lawitz E, Shankar RR, Chaudhri E, Liu J, Lam RLH, et al. A phase IIa active-comparator-controlled study to evaluate the efficacy and safety of efinopegdutide in patients with non-alcoholic fatty liver disease. J Hepatol. 2023;79:888–97.
Rosenstock J, Wysham C, Frías JP, Kaneko S, Lee CJ, Fernández Landó L, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021;398:143–55.
Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387:205–16.
Garvey WT, Frias JP, Jastreboff AM, le Roux CW, Sattar N, Aizenberg D, et al. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2023;402:613–26.
Loomba R, Hartman ML, Lawitz EJ, Vuppalanchi R, Boursier J, Bugianesi E, et al. Tirzepatide for metabolic dysfunction-associated steatohepatitis with liver fibrosis. N Engl J Med. 2024;391:299–310.
Boland ML, Laker RC, Mather K, Nawrocki A, Oldham S, Boland BB, et al. Resolution of NASH and hepatic fibrosis by the GLP-1R/GcgR dual-agonist cotadutide via modulating mitochondrial function and lipogenesis. Nat Metab. 2020;2:413–31.
Frías JP, Davies MJ, Rosenstock J, Pérez Manghi FC, Fernández Landó L, Bergman BK, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385:503–15.
Sanyal A, Frias JP, Thomas MK, Mather KJ, Wu QW, Du Y, et al. Triple hormone receptor agonist retatrutide resolves steatosis in > 85% of subjects with MASLD and obesity in association with improved metabolic health. Hepatology. 2023;78:S154–5.
Jastreboff AM, Kaplan LM, Frías JP, Wu Q, Du Y, Gurbuz S, et al. Triple-hormone-receptor agonist retatrutide for obesity - a phase 2 trial. N Engl J Med. 2023;389:514–26.
Coskun T, Urva S, Roell WC, Qu H, Loghin C, Moyers JS, et al. LY3437943, a novel triple glucagon, GIP, and GLP-1 receptor agonist for glycemic control and weight loss: from discovery to clinical proof of concept. Cell Metab. 2022;34:1234–47. e9
Sodhi M, Rezaeianzadeh R, Kezouh A, Etminan M. Risk of gastrointestinal adverse events associated with glucagon-like peptide-1 receptor agonists for weight loss. JAMA. 2023;330:1795–7.
Kliewer SA, Mangelsdorf DJ. Bile acids as hormones: the FXR-FGF15/19 pathway. Dig Dis. 2015;33:327–31.
Harrison SA, Neff G, Guy CD, Bashir MR, Paredes AH, Frias JP, et al. Efficacy and safety of aldafermin, an engineered FGF19 analog, in a randomized, double-blind, placebo-controlled trial of patients with nonalcoholic steatohepatitis. Gastroenterology. 2021;160:219–31. e1
Luo J, Ko B, Elliott M, Zhou M, Lindhout DA, Phung V, et al. A nontumorigenic variant of FGF19 treats cholestatic liver diseases. Sci Transl Med. 2014;6:247ra100.
Lan T, Morgan DA, Rahmouni K, Sonoda J, Fu X, Burgess SC, et al. FGF19, FGF21, and an FGFR1/beta-Klotho-activating antibody act on the nervous system to regulate body weight and glycemia. Cell Metab. 2017;26:709–18. e3
Harrison SA, Abdelmalek MF, Neff G, Gunn N, Guy CD, Alkhouri N, et al. Aldafermin in patients with non-alcoholic steatohepatitis (ALPINE 2/3): a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Gastroenterol Hepatol. 2022;7:603–16.
Rinella ME, Lieu HD, Kowdley KV, Goodman ZD, Alkhouri N, Lawitz E, et al. A randomized, double-blind, placebo-controlled trial of aldafermin in patients with NASH and compensated cirrhosis. Hepatology. 2024;79:674–89.
BonDurant LD, Potthoff MJ. Fibroblast growth factor 21: a versatile regulator of metabolic homeostasis. Annu Rev Nutr. 2018;38:173–96.
Harrison SA, Ruane PJ, Freilich BL, Neff G, Patil R, Behling CA, et al. Efruxifermin in non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled, phase 2a trial. Nat Med. 2021;27:1262–71.
Szczepanska E, Gietka-Czernel M. FGF21: A novel regulator of glucose and lipid metabolism and whole-body energy balance. Horm Metab Res. 2022;54:203–11.
Harrison SA, Frias JP, Neff G, Abrams GA, Lucas KJ, Sanchez W, et al. Safety and efficacy of once-weekly efruxifermin versus placebo in non-alcoholic steatohepatitis (HARMONY): a multicentre, randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Gastroenterol Hepatol. 2023;8:1080–93.
Harrison SA, Frias JP, Neff G, Abrams G, Lucas KJ, Sanchez W, et al. LBO-002 Efruxifermin significantly reduced liver fibrosis in MASH patients with F2-F3 fibrosis, with sustained improvement in liver injury and resolution of steatohepatitis over 96 weeks (HARMONY phase 2b study). J Hepatol. 2024;80:S8.
Harrison SA. Efruxifermin in compensated cirrhosis due to NASH/MASH: results from a randomized, double-blind, placebo-controlled, phase 2b trial (SYMMETRY). 2023; https://www.natap.org/2023/AASLD/AASLD_104.htm.
Harrison SA, Neff GW, Lucas KJ, Rodriguez M, Wofford S, Benun J, et al. Efruxifermin in compensated cirrhosis due to NASH/MASH: results from a randomized, double-blind, placebo-controlled, phase 2b trial (SYMMETRY). Hepatology. 2024;79:E37–9.
Loomba R, Sanyal AJ, Kowdley KV, Bhatt DL, Alkhouri N, Frias JP, et al. Randomized, controlled trial of the FGF21 analogue pegozafermin in NASH. N Engl J Med. 2023;389:998–1008.
Loomba R, Sanyal A, Kowdley KV, Bhatt DL, Alkhouri N, Frias JP, et al. Fibrosis improvement with pegozafermin treatment in MASH patients with F4 fibrosis: analysis from a 24-week randomized, double-blind, placebo-controlled phase 2 trial (ENLIVEN). Hepatology. 2023;78:S3–8.
Wei W, Dutchak PA, Wang X, Ding X, Wang X, Bookout AL, et al. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor γ. Proc Natl Acad Sci USA. 2012;109:3143–8.
Kim AM, Somayaji VR, Dong JQ, Rolph TP, Weng Y, Chabot JR, et al. Once-weekly administration of a long-acting fibroblast growth factor 21 analogue modulates lipids, bone turnover markers, blood pressure and body weight differently in obese people with hypertriglyceridaemia and in non-human primates. Diabetes Obes Metab. 2017;19:1762–72.
Neuschwander-Tetri BA. Farnesoid X receptor agonists: what they are and how they might be used in treating liver disease. Curr Gastroenterol Rep. 2012;14:55–62.
Jiang L, Zhang H, Xiao D, Wei H, Chen Y. Farnesoid X receptor (FXR): structures and ligands. Comput Struct Biotechnol J. 2021;19:2148–59.
Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394:2184–96.
Sanyal AJ, Ratziu V, Loomba R, Anstee QM, Kowdley KV, Rinella ME, et al. Results from a new efficacy and safety analysis of the REGENERATE trial of obeticholic acid for treatment of pre-cirrhotic fibrosis due to non-alcoholic steatohepatitis. J Hepatol. 2023;79:1110–20.
Harrison SA, Gunn N, Neff GW, Kohli A, Liu L, Flyer A, et al. A phase 2, proof of concept, randomised controlled trial of berberine ursodeoxycholate in patients with presumed non-alcoholic steatohepatitis and type 2 diabetes. Nat Commun. 2021;12:5503.
Santos VN, Lanzoni VP, Szejnfeld J, Shigueoka D, Parise ER. A randomized double-blind study of the short-time treatment of obese patients with nonalcoholic fatty liver disease with ursodeoxycholic acid. Braz J Med Biol Res. 2003;36:723–9.
Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C, et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10:1344–51.
Yin J, Xing H, Ye J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism. 2008;57:712–7.
Ratziu V, de Ledinghen V, Oberti F, Mathurin P, Wartelle-Bladou C, Renou C, et al. A randomized controlled trial of high-dose ursodesoxycholic acid for nonalcoholic steatohepatitis. J Hepatol. 2011;54:1011–9.
Harrison SA, Gunn N, Neff GW, Flyer A, Liberman A, MacConell L. Improvements in liver fibroinflammation (as assessed by corrected T1 [cT1]) with HTD1801 (berberine ursodeoxycholate) treatment in patients with non-alcoholic steatohepatitis and type 2 diabetes mellitus. J Hepatol. 2023;78:S653–4.
Kimura I, Ichimura A, Ohue-Kitano R, Igarashi M. Free fatty acid receptors in health and disease. Physiol Rev. 2020;100:171–210.
Secor JD, Fligor SC, Tsikis ST, Yu LJ, Puder M. Free fatty acid receptors as mediators and therapeutic targets in liver disease. Front Physiol. 2021;12:656441.
Jin C, Chen H, Xie L, Zhou Y, Liu LL, Wu J. GPCRs involved in metabolic diseases: pharmacotherapeutic development updates. Acta Pharmacol Sin. 2024;45:1321–36.
Fraser DA, Harrison SA, Schuppan D. Icosabutate: targeting metabolic and inflammatory pathways for the treatment of NASH. Expert Opin Investig Drugs. 2022;31:1269–78.
Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–98.
Tunaru S, Bonnavion R, Brandenburger I, Preussner J, Thomas D, Scholich K, et al. 20-HETE promotes glucose-stimulated insulin secretion in an autocrine manner through FFAR1. Nat Commun. 2018;9:177.
Fraser DA, Wang X, Lund J, Nikolic N, Iruarrizaga-Lejarreta M, Skjaeret T, et al. A structurally engineered fatty acid, icosabutate, suppresses liver inflammation and fibrosis in NASH. J Hepatol. 2022;76:800–11.
Harrison SA, Alkhouri N, Benun J, Ortiz-Lasanta G, Rudraraju M, Steineger HH, et al. Icosabutate in NASH/MASH with fibrosis: results from a randomised, multicentre, double-blind, placebo controlled, phase 2b trial (ICONA). Hepatology. 2024;79:E44–5.
Lacy BE, Levy LC. Lubiprostone: a chloride channel activator. J Clin Gastroenterol. 2007;41:345–51.
Kessoku T, Kobayashi T, Imajo K, Tanaka K, Yamamoto A, Takahashi K, et al. Endotoxins and non-alcoholic fatty liver disease. Front Endocrinol. 2021;12:770986.
Kim MY, Lee SJ, Randolph G, Han YH. Lubiprostone significantly represses fatty liver diseases via induction of mucin and HDL release in mice. Life Sci. 2022;311:121176.
El-Kassas M, Liu HQ, Lee SS. Lubiprostone reduces fat content on MRI-PDFF in patients with MASLD. Hepatology. 2023;78:S152.
Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65:1038–48.
Noureddin M, Alkhouri N, Lawitz E, Kowdley KV, Loomba R, Sanchez W, et al. Topline results from a 12-week phase 2a trial (DUET) evaluating TERN-501, a highly selective thyroid hormone receptor (THR) beta agonist, either as monotherapy or in combination with TERN-101, a nonsteroidal farnesoid X receptor (FXR) agonist, demonstrated significant reductions in MR-based liver fat content and fibro-inflammation in patients with presumed MASH. Hepatology. 2024;79:E33.
Chinese Society of Hepatology, Chinese Medical Association. [Guidelines for the prevention and treatment of metabolic dysfunction-associated (non-alcoholic) fatty liver disease (Version 2024)].Zhonghua Gan Zang Bing Za Zhi. 2024;32:418–34.
Zou H, Ge Y, Lei Q, Ung COL, Ruan Z, Lai Y, et al. Epidemiology and disease burden of non-alcoholic steatohepatitis in greater China: a systematic review. Hepatol Int. 2022;16:27–37.
Harris JM, Martin NE, Modi M. Pegylation: a novel process for modifying pharmacokinetics. Clin Pharmacokinet. 2001;40:539–51.
Qi J, Guo Z, Zhu S, Jiang X, Wu Y, Chen Y, et al. Therapeutic effect of long-acting FGF21 with controlled site-specific modification on nonalcoholic steatohepatitis. Int J Biol Macromol. 2024;261:129797.
Teufel A, Itzel T, Erhart W, Brosch M, Wang XY, Kim YO, et al. Comparison of gene expression patterns between mouse models of nonalcoholic fatty liver disease and liver tissues from patients. Gastroenterology. 2016;151:513–25.e0.
McLaren DG, Han S, Murphy BA, Wilsie L, Stout SJ, Zhou H, et al. DGAT2 inhibition alters aspects of triglyceride metabolism in rodents but not in non-human primates. Cell Metab. 2018;27:1236–48.e6.
Vali Y, Lee J, Boursier J, Petta S, Wonders K, Tiniakos D, et al. Biomarkers for staging fibrosis and non-alcoholic steatohepatitis in non-alcoholic fatty liver disease (the LITMUS project): a comparative diagnostic accuracy study. Lancet Gastroenterol Hepatol. 2023;8:714–25.
Shah A, MacConell L, Shapiro D. Histologic endpoints in NASH clinical trials: the emperor has no clothes. Hepatology. 2022;76:S96–7.
Mangia A, Loomba R, Schattenberg J, Taub R, Labriola D, Noureddin M, et al. Relationship of non-invasive measures with histological response in patients with nonalcoholic steatohepatitis and fibrosis: 52-week data from the phase 3 MAESTRO-NASH trial. Dig Liver Dis. 2024;56:S14.
Keating SE, Chawla Y, De A, George ES. Lifestyle intervention for metabolic dysfunction-associated fatty liver disease: a 24-h integrated behavior perspective. Hepatol Int. 2024; https://doi.org/10.1007/s12072-024-10663-9.
Acknowledgements
The work is supported partially by the National Natural Science Foundation of China (NSFC #82370625, 82170624).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wong, S.W., Yang, Yy., Chen, H. et al. New advances in novel pharmacotherapeutic candidates for the treatment of metabolic dysfunction-associated steatohepatitis (MASH) between 2022 and 2024. Acta Pharmacol Sin 46, 1145–1155 (2025). https://doi.org/10.1038/s41401-024-01466-7
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-024-01466-7
Keywords
This article is cited by
-
Combination of GLP-1 receptor agonist and Akkermansia muciniphila Akk11 reduces adiposity and ameliorates MASLD in T2D mice
Cell & Bioscience (2026)
-
Resmetirom, the first FDA-approved drug for MASH: From drug discovery and action mechanisms to clinical trials
Archives of Pharmacal Research (2025)