Abstract
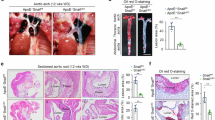
The monocyte adhesion to vascular endothelial cells constitutes a key step in atherosclerosis pathogenesis. We previously found that ROS-autophagy pathway participated in the monocyte-endothelial cell adhesion induced by angiotensin domain type 1 receptor-associated proteins (APJ) and its endogenous ligand apelin-13. In this study, we investigated what specific type of autophagy apelin-13 regulated in this process. By conducting full-scale transcriptomic analysis in apelin-13-treated human umbilical vein endothelial cells (HUVECs), we found that the transcription levels of ER-phagy receptor protein SEC62 were significantly elevated. Importantly, SEC62 was also upregulated in human atherosclerotic lesions. Thus, we investigated the effects of SEC62-dependent ER-phagy on apelin-13-induced monocyte-endothelial cell adhesion and atherosclerosis pathogenesis. We demonstrated that Apelin-13 (0.001−1 μM) dose-dependently upregulated SEC62 expression thereby inducing ER-phagy in HUVECs. This effect was reversed by autophagy inhibitor 3MA (10 mM) and endoplasmic reticulum stress inhibitor salubrinal (10 μM). The siRNA-Sec62, 3MA (10 mM), and salubrinal (10 μM) all inhibited apelin-13-induced monocyte-endothelial cells adhesion, whereas vascular endothelial cells specific SEC62 deletion alleviated atherosclerotic plaques area, intercellular adhesion molecules expression and lesional macrophages in apelin-13-treated APOE−/− mice with high-fat and high-cholesterol diet. Moreover, we demonstrated that ubiquitin-like modification of ALDH1L1 was involved in SEC62-dependent ER-phagy in apelin-13-treated HUVECs: apelin-13 upregulated small ubiquitin-like protein UBL4A, which mediated the ubiquitination-like modification of ALDH1L1 at 812-lysine site. This, in turn, promoted insertion of ALDH1L1 into ER membrane and led to SEC62-dependent ER-phagy. We showed that siRNA-UBL4A, siRNA-ALDH1L1, siRNA-ASNA1, and the mutant of 812 lysine site of ALDH1L1 all decreased apelin-13-induced monocyte-endothelial cell adhesion. We conclude that apelin-13 induces SEC62-dependent ER-phagy to promote monocyte-endothelial cell adhesion and atherosclerosis. This study reveals new mechanisms underlying atherosclerosis and identifies a potential therapeutic target.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
O’Dowd BF, Heiber M, Chan A, Heng HH, Tsui LC, Kennedy JL, et al. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene. 1993;136:355–60.
Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251:471–6.
Pitkin SL, Maguire JJ, Kuc RE, Davenport AP. Modulation of the apelin/APJ system in heart failure and atherosclerosis in man. Br J Pharmacol. 2010;160:1785–95.
He L, Zhou Q, Huang Z, Xu J, Zhou H, Lv D, et al. PINK1/Parkin-mediated mitophagy promotes apelin-13-induced vascular smooth muscle cell proliferation by AMPKalpha and exacerbates atherosclerotic lesions. J Cell Physiol. 2019;234:8668–82.
Lv D, Li H, Chen L. Apelin and APJ, a novel critical factor and therapeutic target for atherosclerosis. Acta Biochim Biophys Sin. 2013;45:527–33.
Li X, Zhang X, Li F, Chen L, Li L, Qin X, et al. 14-3-3 mediates apelin-13-induced enhancement of adhesion of monocytes to human umbilical vein endothelial cells. Acta Biochim Biophys Sin. 2010;42:403–9.
Lu Y, Zhu X, Liang GX, Cui RR, Liu Y, Wu SS, et al. Apelin-APJ induces ICAM-1, VCAM-1 and MCP-1 expression via NF-kappaB/JNK signal pathway in human umbilical vein endothelial cells. Amino Acids. 2012;43:2125–36.
Fumagalli F, Noack J, Bergmann TJ, Presmanes EC, Pisoni GB, Fasana E, et al. Translocon component Sec62 acts in endoplasmic reticulum turnover during stress recovery. Nat Cell Biol. 2016;18:1173–84.
de Boer S, Baran Y, Garcia-Garcia HM, Eskin I, Lenzen MJ, Kleber ME, et al. The European Collaborative Project on inflammation and vascular wall remodeling in atherosclerosis—intravascular ultrasound (ATHEROREMO-IVUS) study. Eurointervention. 2018;14:194–203.
Perrotta I. ER-phagy in human atherosclerosis: an exploratory ultrastructural study. Ultrastruct Pathol. 2020;44:489–95.
Liu MQ, Li HN, Zhou Q, Zhao H, Lv DG, Cao JG, et al. ROS-Autophagy pathway mediates monocytes-human umbilical vein endothelial cells adhesion induced by apelin-13. J Cell Physiol. 2018;233:6839–50.
Lavarone F, Di Lorenzota G, Settembre C. Regulatory events controlling ER-phagy. Curr Opin Cell Biol. 2022;76:102084.
Chen Z, Zhou QL, Chen J, Yang YY, Chen W, Mao H, et al. MCU-dependent mitochondrial calcium uptake-induced mitophagy contributes to apelin-13-stimulated VSMCs proliferation. Vasc Pharmacol. 2022;144:106979.
Zhang Z, Zhang L, Zhou L, Lei YL, Zhang YY, Huang CH. Redox signaling and unfolded protein response coordinate cell fate decisions under ER stress. Redox Biol. 2019;25:101047.
Cao J, Liu Z, Xu Q, Shi R, Zhang G. Research progress in NADPH oxidase family in cardiovascular diseases. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2019;44:1258–67.
Fan J, Ye JB, Kamphorst JJ, Shlomi T, Thompson CB, Rabinowitz JD. Quantitative flux analysis reveals folate-dependent NADPH production. Nature. 2014;510:298–302.
Chartron JW, VanderVelde DG, Clemons WM. Structures of the Sgt2/SGTA dimerization domain with the Get5/UBL4A UBL domain reveal an interaction that forms a conserved dynamic interface. Cell Rep. 2012;2:1620–32.
Liu MQ, Chen Z, Chen LX. Endoplasmic reticulum stress: a novel mechanism and therapeutic target for cardiovascular diseases. Acta Pharmacol Sin. 2016;37:425–43.
Xu Y, Liu YF, Lee JG, Ye YH. A ubiquitin-like domain recruits an oligomeric chaperone to a retrotranslocation complex in endoplasmic reticulum-associated degradation. J Biol Chem. 2013;288:18068–76.
Wilson MP, Durin Z, Unal Ö, Ng BG, Marrecau T, Keldermans L, et al. CAMLG-CDG: a novel congenital disorder of glycosylation linked to defective membrane trafficking. Hum Mol Genet. 2022;31:2571–81.
Xie F, Wu D, Huang SF, Cao JG, Li HN, He L, et al. The endoplasmic reticulum stress-autophagy pathway is involved in apelin-13-induced cardiomyocyte hypertrophy. Acta Pharmacol Sin. 2017;38:1589–600.
Acknowledgements
This work was supported by the grants from the National Natural Science Foundation of China (Grant number: 81603108), Natural Science Foundation of Hunan Province (Grant number: 2023JJ30555), Natural Science Foundation of Guangdong Province (Guangdong Natural Science Foundation, 2023A1515010295) and Health Research Project of Hunan Provincial Health Commission (202103011014).
Author information
Authors and Affiliations
Contributions
LXC and LFL designed research, administered, and supervised the project. ZC, JC, and JLY the experimental parts of the research. XND and JWC generated and characterized the mouse models. QZ and LLW analyzed data. ZC and JGC wrote the paper. LXC and XDX checked the content and language of manuscript. All authors commented on and approved the manuscript for submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Z., Cheng, J., Zhou, Q. et al. SEC62-dependent ER-phagy contributes to apelin-13/APJ-induced monocyte-vascular endothelial cell adhesion in atherosclerosis pathogenesis. Acta Pharmacol Sin 46, 1652–1663 (2025). https://doi.org/10.1038/s41401-024-01471-w
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-024-01471-w
Keywords
This article is cited by
-
Harnessing miRNA therapeutics: a novel approach to combat heart and brain infarctions in atherosclerosis
Cell Death Discovery (2025)