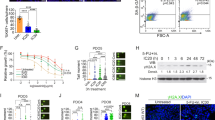
Abstract
Phenethyl isothiocyanate (PEITC) derived from cruciferous vegetables has shown anticancer activities by modulating apoptosis, cell cycle arrest, drug-metabolizing enzymes and even preferentially restoring a ‘WT-like’ conformation to p53R175H. But its molecular anti-cancer mechanisms are not well understood. Evidence shows that switching YAP-binding partners from pro-tumorigenic to pro-apoptotic proteins might hold great potential for the treatment of human cancers harboring mtp53. In this study we investigated the impact of PEITC on mtp53-YAP-p73 interaction in cancers harboring a variety of p53 mutants, but not limited to structural mutations. We showed that breast cancer, colorectal and lung cancer cells harboring mtp53 (p53R280K, p53R273H) were more sensitive to PEITC than those cells harboring wtp53. We demonstrated that PEITC bound to YAP at its WW binding domain, and induced a conformational change, facilitated the dissociation of YAP-mtp53 complex and inhibited their pro-proliferative transcriptional activity in different cancer cells harboring mtp53. Concomitantly, PEITC acted as a molecular glue to enhance the association of YAP-p73 complex and induced apoptosis. These results provide insights into the anticancer activity of PEITC against a wide spectrum of cancers and highlight a unique mode of action for PEITC-based cancer therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Data generated in this study are available upon reasonable request from the corresponding authors.
References
Baugh EH, Ke H, Levine AJ, Bonneau RA, Chan CS. Why are there hotspot mutations in the TP53 gene in human cancers? Cell Death Differ. 2018;25:154–60.
Levine AJ. p53: 800 million years of evolution and 40 years of discovery. Nat Rev Cancer. 2020;20:471–80.
Bargonetti J, Prives C. Gain-of-function mutant p53: history and speculation. J Mol Cell Biol. 2019;11:605–9.
Hassin O, Oren M. Drugging p53 in cancer: one protein, many targets. Nat Rev Drug Discov. 2023;22:127–44.
Peuget S, Zhou X, Selivanova G. Translating p53-based therapies for cancer into the clinic. Nat Rev Cancer. 2024;24:192–215.
Bykov VJN, Eriksson SE, Bianchi J, Wiman KG. Targeting mutant p53 for efficient cancer therapy. Nat Rev Cancer. 2018;18:89–102.
Durairaj G, Demir O, Lim B, Baronio R, Tifrea D, Hall LV, et al. Discovery of compounds that reactivate p53 mutants in vitro and in vivo. Cell Chem Biol. 2022;29:1381–95.e13
Ferraiuolo M, Verduci L, Blandino G, Strano S. Mutant p53 protein and the Hippo transducers YAP and TAZ: a critical oncogenic node in human cancers. Int J Mol Sci. 2017;18:961.
Morciano G, Vezzani B, Missiroli S, Boncompagni C, Pinton P, Giorgi C. An updated understanding of the role of YAP in driving oncogenic responses. Cancers. 2021;13:3100.
Downward J, Basu S. YAP and p73: a complex affair. Mol Cell. 2008;32:749–50.
Levy D, Adamovich Y, Reuven N, Shaul Y. Yap1 phosphorylation by c-Abl is a critical step in selective activation of proapoptotic genes in response to DNA damage. Mol Cell. 2008;29:350–61.
Matallanas D, Romano D, Yee K, Meissl K, Kucerova L, Piazzolla D, et al. RASSF1A elicits apoptosis through an MST2 pathway directing proapoptotic transcription by the p73 tumor suppressor protein. Mol Cell. 2007;27:962–75.
Escoll M, Gargini R, Cuadrado A, Anton IM, Wandosell F. Mutant p53 oncogenic functions in cancer stem cells are regulated by WIP through YAP/TAZ. Oncogene. 2017;36:3515–27.
Di Agostino S, Sorrentino G, Ingallina E, Valenti F, Ferraiuolo M, Bicciato S, et al. YAP enhances the pro-proliferative transcriptional activity of mutant p53 proteins. EMBO Rep. 2016;17:188–201.
Strano S, Munarriz E, Rossi M, Cristofanelli B, Shaul Y, Castagnoli L, et al. Physical and functional interaction between p53 mutants and different isoforms of p73. J Biol Chem. 2000;275:29503–12.
Gaiddon C, Lokshin M, Ahn J, Zhang T, Prives C. A subset of tumor-derived mutant forms of p53 down-regulate p63 and p73 through a direct interaction with the p53 core domain. Mol Cell Biol. 2001;21:1874–87.
Ranjan A, Ramachandran S, Gupta N, Kaushik I, Wright S, Srivastava S, et al. Role of phytochemicals in cancer prevention. Int J Mol Sci. 2019;20:4981.
Ioannides C, Konsue N. A principal mechanism for the cancer chemopreventive activity of phenethyl isothiocyanate is modulation of carcinogen metabolism. Drug Metab Rev. 2015;47:356–73.
M Ezzat S, M Merghany R, M Abdel Baki P, Ali Abdelrahim N, M Osman S, A Salem M, et al. Nutritional sources and anticancer potential of phenethyl isothiocyanate: molecular mechanisms and therapeutic insights. Mol Nutr Food Res. 2024;68:e2400063.
Aggarwal M, Saxena R, Sinclair E, Fu Y, Jacobs A, Dyba M, et al. Reactivation of mutant p53 by a dietary-related compound phenethyl isothiocyanate inhibits tumor growth. Cell Death Differ. 2016;23:1615–27.
Yeh YT, Yeh H, Su SH, Lin JS, Lee KJ, Shyu HW, et al. Phenethyl isothiocyanate induces DNA damage-associated G2/M arrest and subsequent apoptosis in oral cancer cells with varying p53 mutations. Free Radic Biol Med. 2014;74:1–13.
Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H, et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–52.
Elgehama A, Wang Y, Yu Y, Zhou L, Chen Z, Wang L, et al. Targeting the PTP1B-Bcr-Abl1 interaction for the degradation of T315I mutant Bcr-Abl1 in chronic myeloid leukemia. Cancer Sci. 2023;114:247–58.
Wang Y, Gao J, Yu Y, Zhou L, Wang M, Xue W, et al. A plant-derived glucocorticoid receptor modulator with potency to attenuate the side effects of glucocorticoid therapy. Br J Pharmacol. 2023;180:194–213.
Aggarwal M, Saxena R, Asif N, Sinclair E, Tan J, Cruz I, et al. p53 mutant-type in human prostate cancer cells determines the sensitivity to phenethyl isothiocyanate induced growth inhibition. J Exp Clin Cancer Res. 2019;38:307.
Hermeking H, Lengauer C, Polyak K, He TC, Zhang L, Thiagalingam S, et al. 14-3-3sigma is a p53-regulated inhibitor of G2/M progression. Mol Cell. 1997;1:3–11.
Sun HL, Men JR, Liu HY, Liu MY, Zhang HS. FOXM1 facilitates breast cancer cell stemness and migration in YAP1-dependent manner. Arch Biochem Biophys. 2020;685:108349.
Chapeau EA, Sansregret L, Galli GG, Chene P, Wartmann M, Mourikis TP, et al. Direct and selective pharmacological disruption of the YAP-TEAD interface by IAG933 inhibits Hippo-dependent and RAS-MAPK-altered cancers. Nat Cancer. 2024;5:1102–20.
Strano S, Munarriz E, Rossi M, Castagnoli L, Shaul Y, Sacchi A, et al. Physical interaction with Yes-associated protein enhances p73 transcriptional activity. J Biol Chem. 2001;276:15164–73.
Reggiani F, Gobbi G, Ciarrocchi A, Sancisi V. YAP and TAZ are not identical twins. Trends Biochem Sci. 2021;46:154–68.
Wang X, Di Pasqua AJ, Govind S, McCracken E, Hong C, Mi L, et al. Selective depletion of mutant p53 by cancer chemopreventive isothiocyanates and their structure-activity relationships. J Med Chem. 2011;54:809–16.
Jang W, Kim T, Koo JS, Kim SK, Lim DS. Mechanical cue-induced YAP instructs Skp2-dependent cell cycle exit and oncogenic signaling. EMBO J. 2017;36:2510–28.
Zhang HT, Gui T, Liu RX, Tong KL, Wu CJ, Li Z, et al. Sequential targeting of YAP1 and p21 enhances the elimination of senescent cells induced by the BET inhibitor JQ1. Cell Death Dis. 2021;12:121.
Jang JW, Kim MK, Lee YS, Lee JW, Kim DM, Song SH, et al. RAC-LATS1/2 signaling regulates YAP activity by switching between the YAP-binding partners TEAD4 and RUNX3. Oncogene. 2017;36:999–1011.
Dewey JA, Delalande C, Azizi SA, Lu V, Antonopoulos D, Babnigg G. Molecular glue discovery: current and future approaches. J Med Chem. 2023;66:9278–96.
Schreiber SL. The rise of molecular glues. Cell. 2021;184:3–9.
Xu J, Reumers J, Couceiro JR, De Smet F, Gallardo R, Rudyak S, et al. Gain of function of mutant p53 by coaggregation with multiple tumor suppressors. Nat Chem Biol. 2011;7:285–95.
Acknowledgements
We thank Dian-hua Chen (School of Life Sciences, Nanjing University) for technological assistance.
Funding
This work was supported by National Natural Science Foundation of China (Grant Nos. 81974504, 82230116), National Key R&D Program of China (Grant No. 2022YFC3500202), and Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (Grant No. ZYYCXTD-C-202208).
Author information
Authors and Affiliations
Contributions
YS and QX designed this study; YXW, LWW, YH, LZ and GYL performed research; YXW, LWW, JWY analyzed data; JCC and XFW contributed to the discussion; YXW, LWW and YS wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
Jing-cai Cheng was in Drug R&D Institute, JC (Wuxi) Company Inc. He declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Yx., Wang, Lw., Huang, Y. et al. Natural compound PEITC inhibits gain of function of p53 mutants in cancer cells by switching YAP-binding partners between p53 and p73. Acta Pharmacol Sin 46, 1722–1732 (2025). https://doi.org/10.1038/s41401-025-01474-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-025-01474-1