Abstract
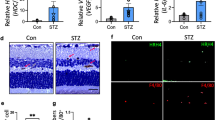
Diabetic retinopathy (DR) is a common and specific microvascular complication of diabetes and the leading cause of blindness in working-age adults. Endothelial-mesenchymal transition (EndoMT) underlies various chronic vascular diseases, while histone deacetylase 9 (HDAC9) is involved in the pathological process of cardiovascular diseases, cerebrovascular diseases, autoimmune diseases, and breast cancer. Recent evidence has shown that HDAC9 promotes EndoMT, thereby affecting the progression of atherosclerotic disease. In this study, we investigated the critical role of HDAC9 in DR and the underlying mechanism. DR model was established in mice by injecting streptozotocin (STZ, 50 mg/kg) for 5 consecutive days. Blood glucose was monitored regularly and DR experiments were performed 12 weeks after modeling. We showed that the expression levels of HDAC9 were significantly elevated in the vitreous fluid of diabetic patients and the retinal endothelial cells of DR model mice. Knockdown of endothelial HDAC9 reduced EndoMT and alleviated DR pathology in vivo, whereas overexpression of HDAC9 exacerbated EndoMT in DR model mice. To elucidate the downstream target genes of HDAC9 implicated in DR, we conducted integrated ChIP-seq and RNA-seq analysis of the retina in STZ-induced retinopathy and established that HDAC9 was involved in the transcriptional regulation of annexin A2 (ANXA2). We demonstrated that HDAC9 was bound to the promoter region of ANXA2, leading to the downregulation of ANXA2 expression in high glucose-treated human retinal microvascular endothelial cells and STZ-induced DR model mice. Overexpression of ANXA2 significantly reduced the EndoMT process in STZ-induced DR model mice. Collectively, our results demonstrate that HDAC9 promotes EndoMT by regulating ANXA2 transcription, thereby disrupting vascular homeostasis during DR. This study sheds light on the roles of HDAC9 and ANXA2 in DR pathology and provides a theoretical foundation for the potential therapeutic strategies to target DR.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81.
Kinuthia UM, Wolf A, Langmann T. Microglia and inflammatory responses in diabetic retinopathy. Front Immunol. 2020;11:564077.
Teo ZL, Tham YC, Yu M, Chee ML, Rim TH, Cheung N, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology. 2021;128:1580–91.
Nawaz IM, Rezzola S, Cancarini A, Russo A, Costagliola C, Semeraro F, et al. Human vitreous in proliferative diabetic retinopathy: characterization and translational implications. Prog Retin Eye Res. 2019;72:100756.
Fong DS, Girach A, Boney A. Visual side effects of successful scatter laser photocoagulation surgery for proliferative diabetic retinopathy: a literature review. Retina. 2007;27:816–24.
Wang W, Lo ACY. Diabetic retinopathy: pathophysiology and treatments. Int J Mol Sci. 2018;19:1816.
Kolluru GK, Bir SC, Kevil CG. Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med. 2012;2012:918267.
Feng B, Chen S, McArthur K, Wu Y, Sen S, Ding Q, et al. miR-146a-Mediated extracellular matrix protein production in chronic diabetes complications. Diabetes. 2011;60:2975–84.
Cao Y, Feng B, Chen S, Chu Y, Chakrabarti S. Mechanisms of endothelial to mesenchymal transition in the retina in diabetes. Invest Ophthalmol Vis Sci. 2014;55:7321–31.
Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20:69–84.
van Meeteren LA, ten Dijke P. Regulation of endothelial cell plasticity by TGF-beta. Cell Tissue Res. 2012;347:177–86.
Piera-Velazquez S, Jimenez SA. Endothelial to mesenchymal transition: role in physiology and in the pathogenesis of human diseases. Physiol Rev. 2019;99:1281–324.
Magrini E, Villa A, Angiolini F, Doni A, Mazzarol G, Rudini N, et al. Endothelial deficiency of L1 reduces tumor angiogenesis and promotes vessel normalization. J Clin Invest. 2014;124:4335–50.
Madar S, Goldstein I, Rotter V. Cancer associated fibroblasts’-more than meets the eye. Trends Mol Med. 2013;19:447–53.
Evrard SM, Lecce L, Michelis KC, Nomura-Kitabayashi A, Pandey G, Purushothaman KR, et al. Endothelial to mesenchymal transition is common in atherosclerotic lesions and is associated with plaque instability. Nat Commun. 2016;7:11853.
Chen PY, Qin L, Baeyens N, Li G, Afolabi T, Budatha M, et al. Endothelial-to-mesenchymal transition drives atherosclerosis progression. J Clin Invest. 2015;125:4514–28.
Mina SG, Huang P, Murray BT, Mahler GJ. The role of shear stress and altered tissue properties on endothelial to mesenchymal transformation and tumor-endothelial cell interaction. Biomicrofluidics. 2017;11:044104.
Piera-Velazquez S, Mendoza FA, Jimenez SA. Endothelial to mesenchymal transition (EndoMT) in the pathogenesis of human fibrotic diseases. J Clin Med. 2016;5:45.
Takagaki Y, Lee SM, Dongqing Z, Kitada M, Kanasaki K, Koya D. Endothelial autophagy deficiency induces IL6-dependent endothelial mesenchymal transition and organ fibrosis. Autophagy. 2020;16:1905–14.
Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–61.
Kadiyala CS, Zheng L, Du Y, Yohannes E, Kao HY, Miyagi M, et al. Acetylation of retinal histones in diabetes increases inflammatory proteins: effects of minocycline and manipulation of histone acetyltransferase (HAT) and histone deacetylase (HDAC). J Biol Chem. 2012;287:25869–80.
Jin Z, Wei W, Huynh H, Wan Y. HDAC9 inhibits osteoclastogenesis via mutual suppression of PPARgamma/RANKL signaling. Mol Endocrinol. 2015;29:730–8.
Zhang Y, Yang Y, Yang F, Liu X, Zhan P, Wu J, et al. HDAC9-mediated epithelial cell cycle arrest in G2/M contributes to kidney fibrosis in male mice. Nat Commun. 2023;14:3007.
Lecce L, Xu Y, V’Gangula B, Chandel N, Pothula V, Caudrillier A, et al. Histone deacetylase 9 promotes endothelial-mesenchymal transition and an unfavorable atherosclerotic plaque phenotype. J Clin Invest. 2021;131:e131178.
Zhu JY, Yao W, Ni XS, Yao MD, Bai W, Yang TJ, et al. Hyperglycemia-regulated tRNA-derived fragment tRF-3001a propels neurovascular dysfunction in diabetic mice. Cell Rep Med. 2023;4:101209.
Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176:1248–64.
Alva JA, Zovein AC, Monvoisin A, Murphy T, Salazar A, Harvey NL, et al. VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev Dyn. 2006;235:759–67.
Karsdal MA, Manon-Jensen T, Genovese F, Kristensen JH, Nielsen MJ, Sand JM, et al. Novel insights into the function and dynamics of extracellular matrix in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2015;308:G807–30.
Cao Q, Rong S, Repa JJ, St Clair R, Parks JS, Mishra N. Histone deacetylase 9 represses cholesterol efflux and alternatively activated macrophages in atherosclerosis development. Arterioscler Thromb Vasc Biol. 2014;34:1871–9.
Yan K, Cao Q, Reilly CM, Young NL, Garcia BA, Mishra N. Histone deacetylase 9 deficiency protects against effector T cell-mediated systemic autoimmunity. J Biol Chem. 2011;286:28833–43.
Salgado E, Bian X, Feng A, Shim H, Liang Z. HDAC9 overexpression confers invasive and angiogenic potential to triple-negative breast cancer cells via modulating microRNA-206. Biochem Biophys Res Commun. 2018;503:1087–91.
Wang Z, Zang C, Cui K, Schones DE, Barski A, Peng W, et al. Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell. 2009;138:1019–31.
Das S, Natarajan R. HDAC9: an inflammatory link in atherosclerosis. Circ Res. 2020;127:824–6.
Neumann P, Jae N, Knau A, Glaser SF, Fouani Y, Rossbach O, et al. The lncRNA GATA6-AS epigenetically regulates endothelial gene expression via interaction with LOXL2. Nat Commun. 2018;9:237.
Liu X, Yang R, Bai W, Xu X, Bi F, Zhu M, et al. Exploring the role of orexin B-sirtuin 1-HIF-1alpha in diabetes-mellitus induced vascular endothelial dysfunction and associated myocardial injury in rats. Life Sci. 2020;254:117041.
Chatterjee TK, Basford JE, Yiew KH, Stepp DW, Hui DY, Weintraub NL. Role of histone deacetylase 9 in regulating adipogenic differentiation and high fat diet-induced metabolic disease. Adipocyte. 2014;3:333–8.
McKinsey TA, Zhang CL, Olson EN. Control of muscle development by dueling HATs and HDACs. Curr Opin Genet Dev. 2001;11:497–504.
Li C, Yu J, Liao D, Su X, Yi X, Yang X, et al. Annexin A2: the missing piece in the puzzle of pathogen-induced damage. Virulence. 2023;14:2237222.
Acknowledgements
This work was supported by the National Natural Science Foundation of China to Hai-bin Dai (82173789, 81573402, and 81773700). The authors thank the Prof Wei-wei Hu, Zhejiang University, and Dr Wen-lu Li, Massachusetts General Hospital for help with preparing the manuscript. The authors thank Shuang-shuang Liu and San-hua Fang from the Core Facilities, Zhejiang University School of Medicine for their technical support in our experiment.
Author information
Authors and Affiliations
Contributions
YB and ZXS conceived and designed the study. YB performed the experiments with assistance from ZXS, HRL, TFW, YJD, and Yan-hong Wang. Yi-hao Wang, LLH, TZ, and WH conducted data collation and statistical analysis. ZTS, JY, and YB collected clinical samples. YB drafted the original manuscript under the guidance of HBD. HBD and GXW are supervising this experiment. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bei, Y., Shen, Zx., Lin, Hr. et al. Endothelial histone deacetylase 9 promotes diabetic retinopathy in mice by regulating endothelial–mesenchymal transition. Acta Pharmacol Sin 46, 2213–2224 (2025). https://doi.org/10.1038/s41401-025-01523-9
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-025-01523-9
Keywords
This article is cited by
-
Molecular mechanisms of bamboo-derived miRNA-mediated gene regulation and dietary adaptation in giant pandas
BMC Genomics (2025)
-
Endothelial mitochondrial-derived vesicles (EMDVs) with retinal targeted homing properties dynamically modulate the eIF2α-ATF4-CHOP signaling pathway and efficiently restore mitochondrial homeostasis in diabetic retina
Journal of Nanobiotechnology (2025)