Abstract
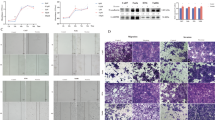
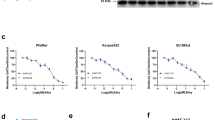
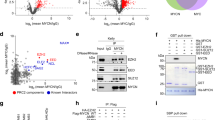
Smoking has been identified as a major risk factor for the development and progression of non-small cell lung cancer (NSCLC). As a key component of tobacco smoke, nicotine is believed to play a significant role in promoting NSCLC growth and progression. EZH2 is an epigenetic regulator highly expressed in the tumor tissues of smokers. However, whether and how nicotine regulates the expression of EZH2 and the underlying mechanisms remain unclear. Bioinformatics analysis and immunohistochemistry were used to compare the expression of EZH2 in NSCLC samples between smokers and nonsmokers. Western blotting, real-time quantitative PCR, and immunofluorescence were employed to confirm the effects of nicotine on EZH2 expression. Cell Counting Kit-8 assays, colony formation assays, 5-ethynyl-2-deoxyuridine staining, and Transwell assays were conducted to analyze the proliferation and metastasis of A549 and H1650 cells treated with siRNA or EZH2 inhibitors. Real-time quantitative PCR and chromatin immunoprecipitation assays were performed to assess the regulatory effect of nicotine on EZH2 transcript levels via c-Myc. Coimmunoprecipitation and ubiquitination assays were used to assess the deubiquitination of c-Myc by OTUB1. Finally, a nude mouse model was used to evaluate the impact of combined c-Myc and EZH2 inhibitors on tumor proliferation and metastasis in vivo. EZH2 is expressed at relatively high levels in NSCLC patients, as determined by both bioinformatic and IHC analyses. Nicotine upregulates EZH2 expression and promotes the proliferation and metastatic ability of lung cancer cells. Inhibition of EZH2 with either DZNep or EPZ6438, EZH2 inhibitors, or siRNA significantly decreased the proliferative and metastatic capacity of NSCLC cells induced by nicotine treatment. Moreover, the study revealed that nicotine induces OTUB1 expression, stabilizes the c-Myc protein via deubiquitination, and enables c-Myc-mediated transcriptional activation of EZH2. Furthermore, the c-Myc inhibitor 10058-F4 exhibited synergistic effects with the EZH2 inhibitor DZNep in suppressing NSCLC cell proliferation and metastasis both in vitro and in vivo.Nicotine regulates the c-Myc/EZH2 signaling pathway via OTUB1-mediated deubiquitination, thereby promoting the proliferation and metastasis of NSCLC cells. This research reveals novel molecular mechanisms of nicotine in the development of NSCLC, providing a theoretical foundation for future therapeutic strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All the data in our study are available upon request.
References
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–63.
Nasim F, Sabath BF, Eapen GA. Lung cancer. Med Clin North Am. 2019;103:463–73.
Nooreldeen R, Bach H. Current and future development in lung cancer diagnosis. Int J Mol Sci. 2021;22:8661.
Tammemagi MC, Berg CD, Riley TL, Cunningham CR, Taylor KL. Impact of lung cancer screening results on smoking cessation. J Natl Cancer Inst. 2014;106:dju084.
Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43:1731–7.
McEvoy CT, Spindel ER. Pulmonary effects of maternal smoking on the fetus and child: effects on lung development, respiratory morbidities, and life long lung health. Paediatr Respir Rev. 2017;21:27–33.
Saint-Andre V, Charbit B, Biton A, Rouilly V, Posseme C, Bertrand A, et al. Smoking changes adaptive immunity with persistent effects. Nature. 2024;626:827–35.
McGrath-Morrow SA, Gorzkowski J, Groner JA, Rule AM, Wilson K, Tanski SE, et al. The effects of nicotine on development. Pediatrics. 2020;145:e20191346.
Milhorn HT Jr. Nicotine dependence. Am Fam Physician. 1989;39:214–24.
Rose JE. Disrupting nicotine reinforcement: from cigarette to brain. Ann N Y Acad Sci. 2008;1141:233–56.
Fan Y, Wang K. Nicotine induces EP4 receptor expression in lung carcinoma cells by acting on AP-2alpha: The intersection between cholinergic and prostanoid signaling. Oncotarget. 2017;8:75854–63.
Ochirbat S, Kan TC, Hsu CC, Huang TH, Chuang KH, Chen M, et al. The angiogenic role of the alpha 9-nicotinic acetylcholine receptor in triple-negative breast cancers. Angiogenesis. 2024;27:827–43.
Deng X. Bcl2 family functions as signaling target in nicotine-/NNK-induced survival of human lung cancer cells. Scientifica. 2014;2014:215426.
Tang MS, Lee HW, Weng MW, Wang HT, Hu Y, Chen LC, et al. DNA damage, DNA repair and carcinogenicity: Tobacco smoke versus electronic cigarette aerosol. Mutat Res Rev Mutat Res. 2022;789:108409.
Wang M, Li Y, Xiao Y, Yang M, Chen J, Jian Y, et al. Nicotine-mediated OTUD3 downregulation inhibits VEGF-C mRNA decay to promote lymphatic metastasis of human esophageal cancer. Nat Commun. 2021;12:7006.
Xiang T, Fei R, Wang Z, Shen Z, Qian J, Chen W. Nicotine enhances invasion and metastasis of human colorectal cancer cells through the nicotinic acetylcholine receptor downstream p38 MAPK signaling pathway. Oncol Rep. 2016;35:205–10.
Cardoso C, Mignon C, Hetet G, Grandchamps B, Fontes M, Colleaux L. The human EZH2 gene: genomic organisation and revised mapping in 7q35 within the critical region for malignant myeloid disorders. Eur J Hum Genet. 2000;8:174–80.
Simon JA, Lange CA. Roles of the EZH2 histone methyltransferase in cancer epigenetics. Mutat Res. 2008;647:21–9.
Kim KH, Roberts CW. Targeting EZH2 in cancer. Nat Med. 2016;22:128–34.
Duan R, Du W, Guo W. EZH2: a novel target for cancer treatment. J Hematol Oncol. 2020;13:104.
Zeng J, Zhang J, Sun Y, Wang J, Ren C, Banerjee S, et al. Targeting EZH2 for cancer therapy: From current progress to novel strategies. Eur J Med Chem. 2022;238:114419.
Kumari K, Das B, Adhya A, Chaudhary S, Senapati S, Mishra SK. Nicotine associated breast cancer in smokers is mediated through high level of EZH2 expression which can be reversed by methyltransferase inhibitor DZNepA. Cell Death Dis. 2018;9:152.
Jin Z, Gao F, Flagg T, Deng X. Tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone promotes functional cooperation of Bcl2 and c-Myc through phosphorylation in regulating cell survival and proliferation. J Biol Chem. 2004;279:40209–19.
Guo M, Li X, Tao W, Teng F, Li C. Vibrio splendidus infection promotes circRNA-FGL1-regulated coelomocyte apoptosis via competitive binding to Myc with the deubiquitinase OTUB1 in Apostichopus japonicus. PLoS Pathog. 2024;20:e1012463.
Xu F, Zang T, Chen H, Zhou C, Wang R, Yu Y, et al. Deubiquitinase OTUB1 regulates doxorubicin-induced cardiotoxicity via deubiquitinating c-MYC. Cell Signal. 2024;113:110937.
Hecht SS, Hatsukami DK. Smokeless tobacco and cigarette smoking: chemical mechanisms and cancer prevention. Nat Rev Cancer. 2022;22:143–55.
Kim K, Picciotto MR. Nicotine addiction: more than just dopamine. Curr Opin Neurobiol. 2023;83:102797.
Wittenberg RE, Wolfman SL, De Biasi M, Dani JA. Nicotinic acetylcholine receptors and nicotine addiction: A brief introduction. Neuropharmacology. 2020;177:108256.
Zhang Q, Ganapathy S, Avraham H, Nishioka T, Chen C. Nicotine exposure potentiates lung tumorigenesis by perturbing cellular surveillance. Br J Cancer. 2020;122:904–11.
Behrens C, Solis LM, Lin H, Yuan P, Tang X, Kadara H, et al. EZH2 protein expression associates with the early pathogenesis, tumor progression, and prognosis of non-small cell lung carcinoma. Clin Cancer Res. 2013;19:6556–65.
Kim NY, Pyo JS. Clinicopathological significance and prognostic role of EZH2 expression in non-small cell lung cancer. Pathol Res Pract. 2017;213:778–82.
Wang B, Liu Y, Luo F, Xu Y, Qin Y, Lu X, et al. Epigenetic silencing of microRNA-218 via EZH2-mediated H3K27 trimethylation is involved in malignant transformation of HBE cells induced by cigarette smoke extract. Arch Toxicol. 2016;90:449–61.
Dang CV. MYC on the path to cancer. Cell. 2012;149:22–35.
Fatma H, Maurya SK, Siddique HR. Epigenetic modifications of c-MYC: role in cancer cell reprogramming, progression and chemoresistance. Semin Cancer Biol. 2022;83:166–76.
Li D, Wang Y, Dong C, Chen T, Dong A, Ren J, et al. CST1 inhibits ferroptosis and promotes gastric cancer metastasis by regulating GPX4 protein stability via OTUB1. Oncogene. 2023;42:83–98.
Liu T, Jiang L, Tavana O, Gu W. The deubiquitylase OTUB1 mediates ferroptosis via stabilization of SLC7A11. Cancer Res. 2019;79:1913–24.
Gong H, Li Y, Yuan Y, Li W, Zhang H, Zhang Z, et al. EZH2 inhibitors reverse resistance to gefitinib in primary EGFR wild-type lung cancer cells. BMC Cancer. 2020;20:1189.
Lee JK, Kim KC. DZNep, inhibitor of S-adenosylhomocysteine hydrolase, down-regulates expression of SETDB1 H3K9me3 HMTase in human lung cancer cells. Biochem Biophys Res Commun. 2013;438:647–52.
Wang L, Chen C, Song Z, Wang H, Ye M, Wang D, et al. EZH2 depletion potentiates MYC degradation inhibiting neuroblastoma and small cell carcinoma tumor formation. Nat Commun. 2022;13:12.
Wang J, Yu X, Gong W, Liu X, Park KS, Ma A, et al. EZH2 noncanonically binds cMyc and p300 through a cryptic transactivation domain to mediate gene activation and promote oncogenesis. Nat Cell Biol. 2022;24:384–99.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 82072595 and 82473191), the Natural Science Foundation of Tianjin (Grant No. 23JCZDJC00710), the Tianjin Key Medical Discipline (Specialty) Construction Project (Grant No. TJYXZDXK-061B), and the Tianjin Health Science and Technology Project (Grant No. TJWJ2022XK005). Beijing Science and Technology Innovation Medical Development Fund (Grant No. KC2023-JX-0288-PZ78).
Author information
Authors and Affiliations
Contributions
This study was designed by JC, HYL, and XBL and conducted by HH, CD, and WHZ. Data analysis was performed by HBZ, ZXZ, YJW, BSL, and YWL, and the manuscript was written by HH, PJC, and XGL. Funding was provided by JC, and the study was supervised by JC, HYL, and YWL. All the authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All experiments were performed in compliance with the relevant regulations, and all patients provided written informed consent. In addition, all animal experiments were performed according to procedures approved by the institutional animal care and use committee of Tianjin Medical University General Hospital. The experiments followed the Guidelines for the Care and Use of Laboratory Animals issued by the Chinese Council on Animal Research. The maximum tumor size allowed by the ethics committee is not more than 2000 mm3, and this requirement was met for all the described experiments.
Consent for publication
All the authors agree with the content of the paper.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, H., Ding, C., Zhao, Wh. et al. Nicotine promotes the progression and metastasis of non-small cell lung cancer by modulating the OTUB1-c-Myc-EZH2 axis. Acta Pharmacol Sin 46, 2509–2521 (2025). https://doi.org/10.1038/s41401-025-01527-5
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-025-01527-5