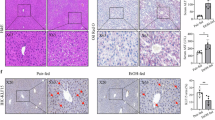
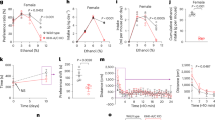
Abstract
Alcohol-related liver disease (ALD) refers to a spectrum of liver diseases caused by chronic or acute excessive alcohol consumption. Excess alcohol consumption and dysregulated immunometabolism are the major contributors to the disease progression. The pathogenesis of ALD remains unclear with macrophage activity playing a key role in disease development. Kv1.3 potassium channel is essential for the regulation of macrophage function. In this study, we investigated whether Kv1.3 channels influenced the progression of ALD by modulating macrophage function. A murine model of ALD was established in male mice. We showed that Kv1.3 knockdown significantly attenuated alcohol-induced liver injury, lipid deposition, and inflammatory responses in ALD mice. Metabolomic analysis revealed that Kv1.3 knockdown significantly increased the levels of tryptamine, a tryptophan metabolite, in the liver of ALD mice. During the last 5 days of the EtOH feeding period, injection of exogenous tryptamine (1, 10, 20 mg·kg−1·d−1, i.p.) notably alleviated ethanol-induced liver steatosis and inflammation in ALD mice. In LPS-challenged RAW264.7 cells, tryptamine (62.5, 125, 250 μM) dose-dependently suppressed the secretion of pro-inflammatory cytokines IL-6, IL-1β and TNF-α. RNA-seq analysis of RAW264.7 cells revealed that tryptamine treatment significantly altered Toll-like receptor and NF-κB signaling pathways. We conclude that in the ALD mice, Kv1.3 negatively regulates tryptamine levels, which inhibits macrophage activation and reduces the inflammatory responses through the TLR4/NF-κB signaling pathway. Our results offer new targets and intervention strategies for the prevention and treatment of ALD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Åberg F, Jiang ZG, Cortez-Pinto H, Männistö V. Alcohol-associated liver disease-Global epidemiology. Hepatology. 2024;80:1307–22.
Leggio L, Lee MR. Treatment of alcohol use disorder in patients with alcoholic liver disease. Am J Med. 2017;130:124–34.
Mackowiak B, Fu Y, Maccioni L, Gao B. Alcohol-associated liver disease. J Clin Invest. 2024;134:e176345.
Louvet A, Mathurin P. Alcoholic liver disease: mechanisms of injury and targeted treatment. Nat Rev Gastroenterol Hepatol. 2015;12:231–42.
Gao H, Jiang Y, Zeng G, Huda N, Thoudam T, Yang Z, et al. Cell-to-cell and organ-to-organ crosstalk in the pathogenesis of alcohol-associated liver disease. eGastroenterology. 2024;2:e100104.
Ambade A, Lowe P, Kodys K, Catalano D, Gyongyosi B, Cho Y, et al. Pharmacological inhibition of CCR2/5 signaling prevents and reverses alcohol-induced liver damage, steatosis, and inflammation in mice. Hepatology. 2019;69:1105–21.
Marin V, Poulsen K, Odena G, McMullen MR, Altamirano J, Sancho-Bru P, et al. Hepatocyte-derived macrophage migration inhibitory factor mediates alcohol-induced liver injury in mice and patients. J Hepatol. 2017;67:1018–25.
Wang M, Shen G, Xu L, Liu X, Brown JM, Feng D, et al. IL-1 receptor-like 1 protects against alcoholic liver injury by limiting NF-κB activation in hepatic macrophages. J Hepatol. 2017;S0168-8278:32263–8.
Wu X, Fan X, McMullen MR, Miyata T, Kim A, Pathak V, et al. Macrophage-derived MLKL in alcohol-associated liver disease: regulation of phagocytosis. Hepatology. 2023;77:902–19.
Van den Bossche J, O’Neill LA, Menon D. Macrophage immunometabolism: where are we (going)? Trends Immunol. 2017;38:395–406.
Liu J, Lv XW, Zhang L, Wang H, Li J, Wu B. Review on biological characteristics of Kv1.3 and its role in liver diseases. Front Pharmacol. 2021;12:652508.
Selvakumar P, Fernández-Mariño AI, Khanra N, He C, Paquette AJ, Wang B, et al. Structures of the T cell potassium channel Kv1.3 with immunoglobulin modulators. Nat Commun. 2022;13:3854.
DeCoursey TE, Chandy KG, Gupta S, Cahalan MD. Voltage-gated K+ channels in human T lymphocytes: a role in mitogenesis? Nature. 1984;307:465–8.
Upadhyay SK, Eckel-Mahan KL, Mirbolooki MR, Tjong I, Griffey SM, Schmunk G, et al. Selective Kv1.3 channel blocker as therapeutic for obesity and insulin resistance. Proc Natl Acad Sci USA. 2013;110:E2239–48.
Lee WH, Najjar SM, Kahn CR, Hinds TD Jr. Hepatic insulin receptor: new views on the mechanisms of liver disease. Metabolism. 2023;145:155607.
Man S, Deng Y, Ma Y, Fu J, Bao H, Yu C, et al. Prevalence of liver steatosis and fibrosis in the general population and various high-risk populations: a nationwide study with 5.7 million adults in China. Gastroenterology. 2023;165:1025–40.
Gao B, Xu MJ, Bertola A, Wang H, Zhou Z, Liangpunsakul S. Animal models of alcoholic liver disease: pathogenesis and clinical relevance. Gene Expr. 2017;17:173–86.
Sougioultzis S, Dalakas E, Hayes PC, Plevris JN. Alcoholic hepatitis: from pathogenesis to treatment. Curr Med Res Opin. 2005;21:1337–46.
Dhanda AD, Collins PL, McCune CA. Is liver biopsy necessary in the management of alcoholic hepatitis? World J Gastroenterol. 2013;19:7825–9.
Navarro-Pérez M, Capera J, Benavente-Garcia A, Cassinelli S, Colomer-Molera M, Felipe A. Kv1.3 in the spotlight for treating immune diseases. Expert Opin Ther Targets. 2024;28:67–82.
Cheng S, Jiang D, Lan X, Liu K, Fan C. Voltage-gated potassium channel 1.3: a promising molecular target in multiple disease therapy. Biomed Pharmacother. 2024;175:116651.
Wu B, Liu JD, Bian E, Hu W, Huang C, Meng X, et al. Blockage of Kv1.3 regulates macrophage migration in acute liver injury by targeting δ-catenin through RhoA signaling. Int J Biol Sci. 2020;16:671–81.
Wu BM, Liu JD, Li YH, Li J. Margatoxin mitigates CCl4‑induced hepatic fibrosis in mice via macrophage polarization, cytokine secretion, and STAT signaling. Int J Mol Med. 2020;45:103–14.
O’Neill LA, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. 2016;16:553–65.
Ayres JS. Immunometabolism of infections. Nat Rev Immunol. 2020;20:79–80.
Makowski L, Chaib M, Rathmell JC. Immunometabolism: from basic mechanisms to translation. Immunol Rev. 2020;295:5–14.
Buck MD, Sowell RT, Kaech SM, Pearce EL. Metabolic instruction of immunity. Cell. 2017;169:570–86.
Ravishankar B, Liu H, Shinde R, Chandler P, Baban B, Tanaka M, et al. Tolerance to apoptotic cells is regulated by indoleamine 2,3-dioxygenase. Proc Natl Acad Sci USA. 2012;109:3909–14.
Krishnan S, Ding Y, Saedi N, Choi M, Sridharan GV, Sherr DH, et al. Gut microbiota-derived tryptophan metabolites modulate inflammatory response in hepatocytes and macrophages. Cell Rep. 2018;23:1099–111.
Agista AZ, Tanuseputero SA, Koseki T, Ardiansyah, Budijanto S, Sultana H, et al. Tryptamine, a microbial metabolite in fermented rice bran suppressed lipopolysaccharide-induced inflammation in a murine macrophage model. Int J Mol Sci. 2022;23:11209.
Williams BB, Van Benschoten AH, Cimermancic P, Donia MS, Zimmermann M, Taketani M, et al. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe. 2014;16:495–503.
Zhai L, Xiao H, Lin C, Wong HLX, Lam YY, Gong M, et al. Gut microbiota-derived tryptamine and phenethylamine impair insulin sensitivity in metabolic syndrome and irritable bowel syndrome. Nat Commun. 2023;14:4986.
Acknowledgements
This study was supported by a grant from the Natural Science Foundation of Anhui Province (grant no. 2108085MH257), Anhui Medical University (Scientific Research Platform Improvement Project of Anhui Medical University (2023xkjT061)), and Research Fund of Anhui Institute of translational medicine (grant no. 2023zhyx-B11). The authors thank BGI Tech Solutions Co., Ltd. and LC-BioTechnology Co., Ltd. (Hangzhou, China) for technical support. The authors also thank LetPub for linguistic assistance during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
BMW, WFT, and LH Conceived and designed the experiments, HW, GQX, TK, and LH Performed the experiments, XXC and WJZ performed the data analyzes and wrote the paper, YYT, SC, CZ, JL, LZ, YTW, DJW helped perform the analysis with constructive discussions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, H., Xia, Gq., Ke, T. et al. Kv1.3 knockdown attenuates alcohol-related liver injury in mice through induction of tryptamine. Acta Pharmacol Sin 46, 2963–2974 (2025). https://doi.org/10.1038/s41401-025-01544-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-025-01544-4