Abstract
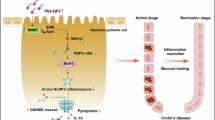
Ulcerative colitis (UC) is a chronic inflammatory bowel disease. The etiology of UC is multifaceted, and the underlying pathogenesis remains incompletely understood. Pyroptosis, programmed cell death mediated by the gasdermins, is a pivotal driver of UC pathology due to its dual role in epithelial barrier disruption and inflammatory amplification. We previously showed that phenethyl isothiocyanate (PEITC), an isothiocyanate derived from cruciferous vegetables, alleviated acute liver injury in mice by suppressing hepatocyte pyroptosis. In this study we evaluated the therapeutic potential of PEITC in the treatment of UC and the underlying mechanisms. UC mouse models were established by administration of 2.5% (w/v) dextran sulfate sodium (DSS) daily for 7 days. PEITC (5, 10, or 20 mg·kg−1·d−1, i.g.) was given 2 days before the start of modeling, and the dosing lasted for a total of 10 days. We showed that during the progression of DSS-induced UC, the pyroptosis pathway was activated accompanied by elevated expression levels of thioredoxin-interacting protein (TXNIP) and NOD-like receptor thermal protein domain associated protein 3 (NLRP3), as well as the activation of caspase-1, gasdermin D (GSDMD) and interleukin-1β (IL-1β). Treatment with PEITC dose-dependently reduced TXNIP and NLRP3 expression while inhibiting the cleavage of proteins associated with the pyroptosis pathway such as caspase-1, GSDMD, and IL-1β. We confirmed the inhibitory effect of PEITC on colonocyte pyroptosis in an in vitro model established in HT29 cells, where PEITC (0.2, 1, 5 µM) dose-dependently inhibited TXNIP and NLRP3 expression and the activation of pro-caspase-1, GSDMD and pro-IL-1β. We revealed that PEITC is directly bound to TXNIP and disrupted the interaction between TXNIP and NLRP3, leading to diminished cellular inflammation and oxidative stress levels. In conclusion, this study demonstrates that PEITC disrupts the interaction of TXNIP and NLRP3 by binding to TXNIP, inhibits NLRP3 activation and colonocyte pyroptosis, and thus effectively alleviates UC symptoms in mice. This study offers novel drug targets along with potential therapeutic candidates for the clinical prevention and treatment of UC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Zhao M, Gonczi L, Lakatos PL, Burisch J. The burden of inflammatory bowel disease in europe in 2020. J Crohns Colitis. 2021;15:1573–87.
Nakase H, Uchino M, Shinzaki S, Matsuura M, Matsuoka K, Kobayashi T, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J Gastroenterol. 2021;56:489–526.
Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG clinical guideline: management of crohn’s disease in adults. Am J Gastroenterol. 2018;113:481–517.
Paramsothy S, Nielsen S, Kamm MA, Deshpande NP, Faith JJ, Clemente JC, et al. Specific bacteria and metabolites associated with response to fecal microbiota transplantation in patients with ulcerative colitis. Gastroenterology. 2019;156:1440–54.e2.
Taylor CT, Colgan SP. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat Rev Immunol. 2017;17:774–85.
Kang Y, Park H, Choe BH, Kang B. The role and function of mucins and its relationship to inflammatory bowel disease. Front Med. 2022;9:848344.
Odenwald MA, Turner JR. The intestinal epithelial barrier: a therapeutic target? Nat Rev Gastroenterol Hepatol. 2017;14:9–21.
Rana N, Privitera G, Kondolf HC, Bulek K, Lechuga S, De Salvo C, et al. GSDMB is increased in IBD and regulates epithelial restitution/repair independent of pyroptosis. Cell. 2022;185:283–98.e17.
Le Berre C, Honap S, Peyrin-Biroulet L. Ulcerative colitis. Lancet. 2023;402:571–84.
Sandborn WJ, Panes J, Sands BE, Reinisch W, Su C, Lawendy N, et al. Venous thromboembolic events in the tofacitinib ulcerative colitis clinical development programme. Aliment Pharmacol Ther. 2019;50:1068–76.
Van Assche G, Manguso F, Zibellini M, Cabriada Nuno JL, Goldis A, Tkachenko E, et al. Oral prolonged release beclomethasone dipropionate and prednisone in the treatment of active ulcerative colitis: results from a double-blind, randomized, parallel group study. Am J Gastroenterol. 2015;110:708–15.
Elias EE, Lyons B, Muruve DA. Gasdermins and pyroptosis in the kidney. Nat Rev Nephrol. 2023;19:337–50.
Newton K, Strasser A, Kayagaki N, Dixit VM. Cell death. Cell. 2024;187:235–56.
Bertheloot D, Latz E, Franklin BS. Necroptosis, pyroptosis and apoptosis: an intricate game of cell death. Cell Mol Immunol. 2021;18:1106–21.
Afonina IS, Zhong Z, Karin M, Beyaert R. Limiting inflammation—the negative regulation of NF-kappaB and the NLRP3 inflammasome. Nat Immunol. 2017;18:861–9.
Zhaolin Z, Guohua L, Shiyuan W, Zuo W. Role of pyroptosis in cardiovascular disease. Cell Prolif. 2019;52:e12563.
Man SM, Karki R, Kanneganti TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev. 2017;277:61–75.
Chen K, Shang S, Yu S, Cui L, Li S, He N. Identification and exploration of pharmacological pyroptosis-related biomarkers of ulcerative colitis. Front Immunol. 2022;13:998470.
Jiang L, Fei D, Gong R, Yang W, Yu W, Pan S, et al. CORM-2 inhibits TXNIP/NLRP3 inflammasome pathway in LPS-induced acute lung injury. Inflamm Res. 2016;65:905–15.
Ji Cho M, Yoon SJ, Kim W, Park J, Lee J, Park JG, et al. Oxidative stress-mediated TXNIP loss causes RPE dysfunction. Exp Mol Med. 2019;51:1–13.
Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–40.
Yu Y, Wu DM, Li J, Deng SH, Liu T, Zhang T, et al. Bixin attenuates experimental autoimmune encephalomyelitis by suppressing TXNIP/NLRP3 inflammasome activity and activating NRF2 signaling. Front Immunol. 2020;11:593368.
Han Y, Xu X, Tang C, Gao P, Chen X, Xiong X, et al. Reactive oxygen species promote tubular injury in diabetic nephropathy: the role of the mitochondrial ROS-TXNIP-NLRP3 biological axis. Redox Biol. 2018;16:32–46.
Jiang N, Liu J, Guan C, Ma C, An J, Tang X. Thioredoxin-interacting protein: a new therapeutic target in bone metabolism disorders? Front Immunol. 2022;13:955128.
Huang S, Tao R, Zhou J, Qian L, Wu J. Trans-10-hydroxy-2-decenoic acid alleviates dextran sulfate sodium-induced colitis in mice via regulating the inflammasome-mediated pyroptotic pathway and enhancing colonic barrier function. Mol Nutr Food Res. 2022;66:e2100821.
Chen Y, Sun L, Liu H, Li J, Guo L, Wang Z. KLF4 interacts with TXNIP to modulate the pyroptosis in ulcerative colitis via regulating NLRP3 signaling. Immun Inflamm Dis. 2024;12:e1199.
Mori N, Shimazu T, Charvat H, Mutoh M, Sawada N, Iwasaki M, et al. Cruciferous vegetable intake and mortality in middle-aged adults: a prospective cohort study. Clin Nutr. 2019;38:631–43.
Wang J, Shi K, An N, Li S, Bai M, Wu X, et al. Direct inhibition of GSDMD by PEITC reduces hepatocyte pyroptosis and alleviates acute liver injury in mice. Front Immunol. 2022;13:825428.
Chikara S, Nagaprashantha LD, Singhal J, Horne D, Awasthi S, Singhal SS. Oxidative stress and dietary phytochemicals: role in cancer chemoprevention and treatment. Cancer Lett. 2018;413:122–34.
Hoch CC, Shoykhet M, Weiser T, Griesbaum L, Petry J, Hachani K, et al. Isothiocyanates in medicine: a comprehensive review on phenylethyl-, allyl-, and benzyl-isothiocyanates. Pharmacol Res. 2024;201:107107.
Cykowiak M, Krajka-Kuzniak V, Kleszcz R, Kucinska M, Szaefer H, Piotrowska-Kempisty H, et al. Comparison of the impact of xanthohumol and phenethyl isothiocyanate and their combination on Nrf2 and NF-kappaB pathways in HepG2 cells in vitro and tumor burden in vivo. Nutrients. 2021;13:3000.
Feng Z, Wang T, Sun Y, Chen S, Hao H, Du W, et al. Sulforaphane suppresses paraquat-induced oxidative damage in bovine in vitro-matured oocytes through Nrf2 transduction pathway. Ecotoxicol Environ Saf. 2023;254:114747.
Wang J, Shi K, Li S, Chen L, Liu W, Wu X, et al. Meisoindigo attenuates dextran sulfate sodium-induced experimental colitis via its inhibition of TAK1 in macrophages. Int Immunopharmacol. 2021;101:108239.
Chen L, Wang J, You Q, He S, Meng Q, Gao J, et al. Activating AMPK to restore tight junction assembly in intestinal epithelium and to attenuate experimental colitis by metformin. Front Pharmacol. 2018;9:761.
Bai M, Li S, Zhang C, An N, Wang J, Qin J, et al. Suppression of neutrophil extracellular traps is responsible for the amelioration of chemotherapeutic intestinal injury by the natural compound PEITC. Toxicol Appl Pharmacol. 2024;484:116857.
Wang J, Bai M, Zhang C, An N, Wan L, Wang XN, et al. Natural compound fraxinellone ameliorates intestinal fibrosis in mice via direct intervention of HSP47-collagen interaction in the epithelium. Acta Pharmacol Sin. 2023;44:2469–78.
Mi L, Hood BL, Stewart NA, Xiao Z, Govind S, Wang X, et al. Identification of potential protein targets of isothiocyanates by proteomics. Chem Res Toxicol. 2011;24:1735–43.
Wangchuk P, Yeshi K, Loukas A. Ulcerative colitis: clinical biomarkers, therapeutic targets, and emerging treatments. Trends Pharmacol Sci. 2024;45:892–903.
Gros B, Kaplan GG. Ulcerative colitis in adults: a review. JAMA. 2023;330:951–65.
De Deo D, Dal Buono A, Gabbiadini R, Spaggiari P, Busacca A, Masoni B, et al. Management of proctitis in ulcerative colitis and the place of biological therapies. Expert Opin Biol Ther. 2024;24:443–53.
Xiao D, Zeng Y, Choi S, Lew KL, Nelson JB, Singh SV. Caspase-dependent apoptosis induction by phenethyl isothiocyanate, a cruciferous vegetable-derived cancer chemopreventive agent, is mediated by Bak and Bax. Clin Cancer Res. 2005;11:2670–9.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 82073910, 82473989, 82230116).
Author information
Authors and Affiliations
Contributions
JW, XFW, and QX designed research. JW, CZ, JQ, NA, and MB performed research and analyzed data. RHD, YS, XDW, JCC, XFW, and QX contributed new reagents. JW and XFW wrote the paper. All of the authors have approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
Jing-cai Cheng is the employee of Drug R&D Institute, JC (Wuxi) Company, Inc. He declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, J., Zhang, C., Qin, J. et al. Direct inhibition of the TXNIP-NLRP3-GSDMD pathway reduces pyroptosis in colonocytes and alleviates ulcerative colitis in mice by the small compound PEITC. Acta Pharmacol Sin 46, 2436–2449 (2025). https://doi.org/10.1038/s41401-025-01549-z
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-025-01549-z