Abstract
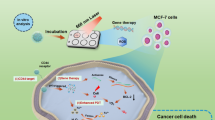
Prodrugs usually convert into active compounds within cells via endogenous or external stimuli to improve the diagnostic accuracy and therapeutic efficacy, but this singular release profile often fails to meet the multifunctional needs of cancer therapeutics. In this study we proposed a strategy of “nanostructural conversion at nano-bio interface” and constructed a small-molecule nanoprodrug (APO-S-Cy7-TCF) for multifunctional anti-tumor phototheranostics. Upon exposure to redox biomolecules (ROS/GSH) in tumor microenvironment, the pristine nanostructure of APO-S-Cy7-TCF disassembled, releasing Cy7-TCF-OH and APO that interacted with heat shock proteins to initiate apoptosis. Cy7-TCF-OH could then re-assemble into smaller nanosaucers with enhanced photothermal properties and self-augmented ROS-generating capacity, enabling synergistic phototherapy for tumor ablation. In particular, Cy7-TCF-OH nanosaucers were long retained in residual tumors and could further interact with albumin to form smaller Cy7-TCF-OH@albumin nanocomposites that time-dependently activated near-infrared fluorescence for prognostic assessment. Using these biomolecule-derived elements to program supramolecular sequential structural conversions at nano-bio interface, our study establishes a new way for small-molecule-based multifunctional phototheranostic platform.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Li H, Wang Y, Tang Q, Yin D, Tang C, He E, et al. The protein corona and its effects on nanoparticle-based drug delivery systems. Acta Biomater. 2021;129:57–72.
Li X, Lovell JF, Yoon J, Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat Rev Clin Oncol. 2020;17:657–74.
Bai G, Yuan P, Cai B, Qiu X, Jin R, Liu S, et al. Stimuli-responsive scaffold for breast cancer treatment combining accurate photothermal therapy and adipose tissue regeneration. Adv Funct Mater. 2019;29:1904401.
Rastinehad AR, Anastos H, Wajswol E, Winoker JS, Sfakianos JP, Doppalapudi SK, et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc Natl Acad Sci USA. 2019;116:18590–6.
Ma G, Liu Z, Zhu C, Chen H, Kwok RTK, Zhang P, et al. H2O2-responsive NIR-II AIE nanobomb for carbon monoxide boosting low-temperature photothermal therapy. Angew Chem Int Ed. 2022;61:e202207213.
Yi X, Duan Q-Y, Wu F-G. Low-temperature photothermal therapy: strategies and applications. Research. 2021;2021:9816594.
Zhao R, Zhu Y, Feng L, Liu B, Hu Y, Zhu H, et al. Architecture of vanadium-based MXene dysregulating tumor redox homeostasis for amplified nanozyme catalytic/photothermal therapy. Adv Mater. 2023;36:2307115.
Li Y, Zhang Y, Dong Y, Akakuru OU, Yao X, Yi J, et al. Ablation of gap junction protein improves the efficiency of nanozyme-mediated catalytic/starvation/mild-temperature photothermal therapy. Adv Mater. 2023;35:2210464.
Dong S, Dong Y, Zhao Z, Liu J, Liu S, Feng L, et al. “Electron transport chain interference” strategy of amplified mild-photothermal therapy and defect-engineered multi-enzymatic activities for synergistic tumor-personalized suppression. J Am Chem Soc. 2023;145:9488–507.
Yang Y, Zhu W, Dong Z, Chao Y, Xu L, Chen M, et al. 1D Coordination polymer nanofibers for low-temperature photothermal therapy. Adv Mater. 2017;29:1703588.
Zhou Z, Yan Y, Hu K, Zou Y, Li Y, Ma R, et al. Autophagy inhibition enabled efficient photothermal therapy at a mild temperature. Biomaterials. 2017;141:116–24.
Wu J, Niu S, Bremner DH, Nie W, Fu Z, Li D, et al. A Tumor microenvironment-responsive biodegradable mesoporous nanosystem for anti-inflammation and cancer theranostics. Adv Health Mater. 2020;9:1901307.
Li W, Peng J, Tan L, Wu J, Shi K, Qu Y, et al. Mild photothermal therapy/photodynamic therapy/chemotherapy of breast cancer by Lyp-1 modified docetaxel/IR820 co-loaded micelles. Biomaterials. 2016;106:119–33.
Chao S, Shen Z, Pei Y, Lv Y, Chen X, Ren J, et al. Pillar[5]arene-based supramolecular photosensitizer for enhanced hypoxic-tumor therapeutic effectiveness. Chem Commun. 2021;57:7625–8.
Jin W, Chen Z, Wang Y, Li J, Li J, Pei Y, et al. Nano metal-photosensitizer based on Aza-BODIPY-Cu complex for CDT-enhanced dual phototherapy. Chin Chem Lett. 2024;35:109328.
Li J, Lv X, Li J, Jin W, Chen Z, Wen Y, et al. A supramolecular near-infrared nanophotosensitizer from host-guest complex of lactose-capped pillar[5]arene with aza-BODIPY derivative for tumor eradication. Org Chem Front. 2023;10:1927–35.
Shu X, Chen Y, Yan P, Xiang Y, Shi Q-Y, Yin T, et al. Biomimetic nanoparticles for effective mild temperature photothermal therapy and multimodal imaging. J Control Release. 2022;347:270–81.
Anselmo AC, Mitragotri S. Nanoparticles in the clinic. Bioeng Transl Med. 2016;1:10–29.
Xiang J, Liu X, Yuan G, Zhang R, Zhou Q, Xie T, et al. Nanomedicine from amphiphilizedprodrugs: Concept and clinical translation. Adv Drug Deliv Rev. 2021;179:114027.
Ma Y, Mou Q, Yan D, Zhu X. Engineering small molecule nanodrugs to overcome barriers for cancer therapy. View. 2020;1:20200062.
Wang J, Li Y, Nie G. Multifunctional biomolecule nanostructures for cancer therapy. Nat Rev Mater. 2021;6:766–83.
Fu S, Li G, Zang W, Zhou X, Shi K, Zhai Y. Pure drug nano-assemblies: A facile carrier-free nanoplatform for efficient cancer therapy. Acta Pharm Sin B. 2022;12:92–106.
Dong X, Brahma RK, Fang C, Yao SQ. Stimulus-responsive self-assembled prodrugs in cancer therapy. Chem Sci. 2022;13:4239–69.
Zhang Y, Cui H, Zhang R, Zhang H, Huang W. Nanoparticulation of prodrug into medicines for cancer therapy. Adv Sci. 2021;8:2101454.
Li G, Sun B, Li Y, Luo C, He Z, Sun J. Small-molecule prodrug nanoassemblies: An emerging nanoplatform for anticancer drug delivery. Small. 2021;17:2101460.
Walther R, Rautio J, Zelikin AN. Prodrugs in medicinal chemistry and enzyme prodrug therapies. Adv Drug Deliv Rev. 2017;118:65–77.
Lepeltier E, Bourgaux C, Couvreur P. Nanoprecipitation and the “Ouzo effect”: Application to drug delivery devices. Adv Drug Deliv Rev. 2014;71:86–97.
Wang D, Liu B, Ma Y, Wu C, Mou Q, Deng H, et al. A molecular recognition approach to synthesize nucleoside analogue based multifunctional nanoparticles for targeted cancer therapy. J Am Chem Soc. 2017;139:14021–4.
Huang P, Wang D, Su Y, Huang W, Zhou Y, Cui D, et al. Combination of small molecule prodrug and nanodrug delivery: amphiphilic drug–drug conjugate for cancer therapy. J Am Chem Soc. 2014;136:11748–56.
Liang M, Mu X, Li Y, Tan Y, Hao X, Tang Y, et al. Heptamethine cyanine-based nanotheranostics with catalase-like activity for synergistic phototherapy of cancer. Adv Funct Mater. 2023;33:2302112.
Feng G, Zhang G-Q, Ding D. Design of superior phototheranostic agents guided by Jablonski diagrams. Chem Soc Rev. 2020;49:8179–234.
Liu Y, Bhattarai P, Dai Z, Chen X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem Soc Rev. 2019;48:2053–108.
Wang Y, Zhu W, Du W, Liu X, Zhang X, Dong H, et al. Cocrystals strategy towards materials for near-infrared photothermal conversion and imaging. Angew Chem Int Ed. 2018;57:3963–7.
Jung HS, Verwilst P, Sharma A, Shin J, Sessler JL, Kim JS. Organic molecule-based photothermal agents: an expanding photothermal therapy universe. Chem Soc Rev. 2018;47:2280–97.
Ko SK, Kim J, Na DC, Park S, Park SH, Hyun JY, et al. A small molecule inhibitor of ATPase activity of HSP70 induces apoptosis and has antitumor activities. Chem Biol. 2015;22:391–403.
Cho HJ, Gee HY, Baek K-H, Ko S-K, Park J-M, Lee H, et al. A small molecule that binds to an ATPase domain of Hsc70 promotes membrane trafficking of mutant cystic fibrosis transmembrane conductance regulator. J Am Chem Soc. 2011;133:20267–76.
Williams DR, Ko S-K, Park S, Lee M-R, Shin I. An apoptosis-inducing small molecule that binds to heat shock protein 70. Angew Chem Int Ed. 2008;47:7466–9.
Chakrabarti S, Michor F. Pharmacokinetics and drug interactions determine optimum combination strategies in computational models of cancer evolution. Cancer Res. 2017;77:3908–21.
Nezhadi S, Dorkoosh FA. Co-delivery systems: hope for clinical application? Drug Deliv Transl Res. 2022;12:1339–54.
Mu X, Wu F, Tang Y, Wang R, Li Y, Li K, et al. Boost photothermal theranostics via self-assembly-induced crystallization (SAIC). Aggregate. 2022;3:e170.
Mu X, Lu Y, Wu F, Wei Y, Ma H, Zhao Y, et al. Supramolecular nanodiscs self-assembled from non-ionic heptamethine cyanine for iImaging-guided cancer photothermal therapy. Adv Mater. 2020;32:1906711.
Mu X, Feng W, Li C, Li K, Li Y, Jing X, et al. Lighting up self-quenching nanoaggregates with protein corona for simultaneous intraoperative imaging and photothermal theranostics of metastatic cancer. Anal Chem. 2022;94:9775–84.
Cui X, Ruan Q, Zhuo X, Xia X, Hu J, Fu R, et al. Photothermal nanomaterials: A powerful light-to-heat converter. Chem Rev. 2023;123:6891–952.
Mu X, Huang Z, Feng W, Zhai M, Wang Y, Zhou D, et al. Zwitterionic rhodamine-CPT prodrug nanoparticles with GSH/H2O2 responsiveness for cancer theranostics. Theranostics. 2023;13:267–77.
Chu B, Qu Y, He X, Hao Y, Yang C, Yang Y, et al. ROS-responsive camptothecin prodrug nanoparticles for on-demand drug release and combination of chemotherapy and photodynamic therapy. Adv Funct Mater. 2020;30:2005918.
Wu F, Lu Y, Mu X, Chen Z, Liu S, Zhou X, et al. Intriguing H-aggregates of heptamethine cyanine for imaging-guided photothermal cancer therapy. ACS Appl Mater Interfaces. 2020;12:32388–96.
Desai N, Trieu V, Damascelli B, Soon-Shiong P. SPARC expression correlates with tumor response to albumin-bound paclitaxel in head and neck cancer patients. Transl Oncol. 2009;2:59–64.
Niu B, Liao K, Zhou Y, Wen T, Quan G, Pan X, et al. Application of glutathione depletion in cancer therapy: Enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials. 2021;277:121110.
Acknowledgements
Financial support was provided by National Natural Science Foundation of China (22071128), Shandong Provincial Natural Science Foundation (ZR2020ZD31, ZR2023QB077), and Qingdao Postdoctoral Foundation (QDBSH20230102053).
Author information
Authors and Affiliations
Contributions
XFZ, SS, and RW conceived and designed the study. SS, RW, and XLEM acquired and analyzed data. WBF and HK collected and analyzed cellular and animal data. YJL and MG interpreted the data. SS, RW and XFZ wrote the paper. HRS interpreted the data and supervised the project. YXL and XFZ acquired funding and supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, S., Wang, R., Mu, Xle. et al. Programmed sequential nanostructural conversion at nano-bio interface for synergistic cancer phototheranostics. Acta Pharmacol Sin 46, 3343–3354 (2025). https://doi.org/10.1038/s41401-025-01609-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-025-01609-4