Abstract
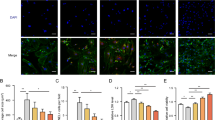
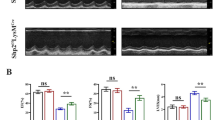
Myocardial remodeling is critical pathological processes in various cardiovascular diseases, where redox imbalance and mitochondrial bioenergetic perturbations emerge as key determinants. Prohibitin 2 (PHB2), which resides in the mitochondrial inner membrane, serves as a critical regulator of mitochondrial homeostasis. In this study we investigated the protective role of PHB2 in transverse aortic constriction (TAC)-induced cardiac remodeling with a particular focus on its ability to safeguard the heart by improving mitochondrial function and alleviating oxidative stress. We revealed that PHB2 expression was significantly decreased in the heart of TAC mice and in Ang II (1 μM)-treated cardiomyocytes. Cardiac-specific PHB2 overexpression mitigated TAC-induced cardiac remodeling, improving cardiac function and attenuating hypertrophy. Additionally, PHB2 overexpression effectively suppressed oxidative stress in the hearts of TAC mice, while improving mitochondrial morphology and the integrity of inner membrane structure. Furthermore, PHB2 overexpression restored mitochondrial function in Ang II-treated cardiomyocytes evidenced by elevated ATP levels and enhanced oxidative phosphorylation capacity. IP-MS analysis revealed that PHB2 directly interacted with Transporter of Outer Mitochondrial Membrane 40 (TOMM40) to regulate mitochondrial function. Importantly, silencing TOMM40 abolished the protective effects of PHB2. We demonstrated that PHB2 preserves TOMM40 protein levels predominantly through inhibition of ubiquitin-dependent proteasomal degradation. Collectively, we discover a new function of PHB2 in safeguarding mitochondrial morphofunctional homeostasis in response to pathological stress through facilitating TOMM40 stabilization, suggesting PHB2 as a promising therapeutic target for potential interventions in heart diseases.

Schematic illustration of PHB2’s potential protective mechanism against cardiac hypertrophy. PHB2 protects against pressure overload-induced cardiac hypertrophy through preserving TOMM40 protein to maintain mitochondrial energetic homeostasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, Coats AJS. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. 2023;118:3272–87.
Ma ZG, Yuan YP, Fan D, Zhang X, Teng T, Song P, et al. IRX2 regulates angiotensin II-induced cardiac fibrosis by transcriptionally activating EGR1 in male mice. Nat Commun. 2023;14:4967.
Mensah GA, Fuster V, Murray CJL, Roth GA. Global Burden of Cardiovascular Diseases and Risks Collaborators. Global burden of cardiovascular diseases and risks, 1990-2022. J Am Coll Cardiol. 2023;82:2350–473.
Ranjbarvaziri S, Kooiker KB, Ellenberger M, Fajardo G, Zhao MM, Vander Roest AS, et al. Altered cardiac energetics and mitochondrial dysfunction in hypertrophic cardiomyopathy. Circulation. 2021;144:1714–31.
Peoples JN, Saraf A, Ghazal N, Pham TT, Kwong JQ. Mitochondrial dysfunction and oxidative stress in heart disease. Exp Mol Med. 2019;51:1–13.
Lopaschuk GD, Karwi QG, Tian R, Wende AR, Abel ED. Cardiac energy metabolism in heart failure. Circ Res. 2021;128:1487–513.
Zhuang LF, Jia KN, Chen C, Li ZG, Zhao JX, Hu J, et al. DYRK1B-STAT3 drives cardiac hypertrophy and heart failure by impairing mitochondrial bioenergetics. Circulation. 2022;145:829–46.
Ren J, Pulakat L, Whaley-Connell A, Sowers JR. Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. J Mol Med (Berl). 2010;88:993–1001.
Bugger H, Schwarzer M, Chen D, Schrepper A, Amorim PA, Schoepe M, et al. Proteomic remodelling of mitochondrial oxidative pathways in pressure overload-induced heart failure. Cardiovasc Res. 2010;85:376–84.
Wu J, Lu J, Huang JY, You JY, Ding ZW, Ma LL, et al. Variations in energy metabolism precede alterations in cardiac structure and function in hypertrophic preconditioning. Front Cardiovasc Med. 2020;7:602100.
Yang D, Liu HQ, Liu FY, Guo Z, An P, Wang MY, et al. Mitochondria in pathological cardiac hypertrophy research and therapy. Front Cardiovasc Med. 2021;8:822969.
Jomova K, Alomar SY, Alwasel SH, Nepovimova E, Kuca K, Valko M. Several lines of antioxidant defense against oxidative stress: antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch Toxicol. 2024;98:1323–67.
Moris D, Spartalis M, Tzatzaki E, Spartalis E, Karachaliou GS, Triantafyllis AS, et al. The role of reactive oxygen species in myocardial redox signaling and regulation. Ann Transl Med. 2017;5:324.
Pham L, Arroum T, Wan J, Pavelich L, Bell J, Morse PT, et al. Regulation of mitochondrial oxidative phosphorylation through tight control of cytochrome c oxidase in health and disease - implications for ischemia/reperfusion injury, inflammatory diseases, diabetes, and cancer. Redox Biol. 2024;78:103426.
Heine KB, Parry HA, Hood WR. How does density of the inner mitochondrial membrane influence mitochondrial performance? Am J Physiol Regul Integr Comp Physiol. 2023;324:R242–R248.
Chapa-Dubocq XR, Rodríguez-Graciani KM, Escobales N, Javadov S. Mitochondrial volume regulation and swelling mechanisms in cardiomyocytes. Antioxidants. 2023;12:1517.
Wei YJ, Chiang WC, Sumpter R, Mishra P, Levine B. Prohibitin 2 is an inner mitochondrial membrane mitophagy receptor. Cell. 2017;168:224–238.e10.
Zheng XH, Liu K, Xie QQ, Xin HK, Chen W, Lin SY, et al. PHB2 alleviates neurotoxicity of prion peptide PrP106-126 via PINK1/Parkin-dependent mitophagy. Int J Mol Sci. 2023;24:15919.
Signorile A, Sgaramella G, Bellomo F, De Rasmo D. Prohibitins: a critical role in mitochondrial functions and implication in diseases. Cells. 2019;8:71.
Hernando-Rodríguez B, Artal-Sanz M. Mitochondrial quality control mechanisms and the PHB (prohibitin) complex. Cells. 2018;7:238.
Winge DR, Tzagoloff A. Assembly of the mitochondrial respiratory chain. Preface. Biochim Biophys Acta. 2009;1793:1.
Jian CS, Xu FL, Hou TT, Sun T, Li JH, Cheng HP, et al. Deficiency of PHB complex impairs respiratory supercomplex formation and activates mitochondrial flashes. J Cell Sci. 2017;130:2620–30.
Anderson CJ, Kahl A, Fruitman H, Qian LP, Zhou P, Manfredi G, et al. Prohibitin levels regulate OMA1 activity and turnover in neurons. Cell Death Differ. 2020;27:1896–906.
Li LZ, Martin-Levilain J, Jiménez-Sánchez C, Karaca M, Foti M, Martinou JC, et al. In vivo stabilization of OPA1 in hepatocytes potentiates mitochondrial respiration and gluconeogenesis in a prohibitin-dependent way. J Biol Chem. 2019;294:12581–98.
Wu DC, Jian CS, Peng Q, Hou TT, Wu KL, Shang BZ, et al. Prohibitin 2 deficiency impairs cardiac fatty acid oxidation and causes heart failure. Cell Death Dis. 2020;11:181.
Zhang N, Zhou ZY, Meng YY, Liao HH, Mou SQ, Lin Z, et al. HINT2 protects against pressure overload-induced cardiac remodelling through mitochondrial pathways. J Cell Mol Med. 2024;28:e18276.
Ji TX, Zhang P, Zhang XJ, Zhao YC, Deng KQ, Jiang X, et al. The ubiquitin E3 ligase TRAF6 exacerbates pathological cardiac hypertrophy via TAK1-dependent signalling. Nat Commun. 2016;7:11267.
Li D, Guo YY, Cen XF, Qiu HL, Chen S, Zeng XF, et al. Lupeol protects against cardiac hypertrophy via TLR4-PI3K-Akt-NF-κB pathways. Acta Pharmacol Sin. 2022;43:1989–2002.
Xie SY, Liu SQ, Zhang T, Shi WK, Xing Y, Fang WX, et al. USP28 serves as a key suppressor of mitochondrial morphofunctional defects and cardiac dysfunction in the diabetic heart. Circulation. 2024;149:684–706.
Ago T, Liu T, Zhai PY, Chen W, Li H, Molkentin JD, et al. A redox-dependent pathway for regulating class II HDACs and cardiac hypertrophy. Cell. 2008;133:978–93.
Wang XD, Zhang GY, Dasgupta S, Niewold EL, Li C, Li QF, et al. ATF4 protects the heart from failure by antagonizing oxidative stress. Circ Res. 2022;131:91–105.
Brown DI, Griendling KK. Regulation of signal transduction by reactive oxygen species in the cardiovascular system. Circ Res. 2015;116:531–49.
Ritterhoff J, Tian R. Metabolic mechanisms in physiological and pathological cardiac hypertrophy: new paradigms and challenges. Nat Rev Cardiol. 2023;20:812–29.
Bertero E, Maack C. Metabolic remodelling in heart failure. Nat Rev Cardiol. 2018;15:457–70.
Ma YL, Kong CY, Guo Z, Wang MY, Wang P, Liu FY, et al. Semaglutide ameliorates cardiac remodeling in male mice by optimizing energy substrate utilization through the Creb5/NR4a1 axis. Nat Commun. 2024;15:4757.
Merkwirth C, Dargazanli S, Tatsuta T, Geimer S, Löwer B, Wunderlich FT, et al. Prohibitins control cell proliferation and apoptosis by regulating OPA1-dependent cristae morphogenesis in mitochondria. Genes Dev. 2008;22:476–88.
Yang MJ, Abudureyimu M, Wang X, Zhou Y, Zhang YM, Ren J. PHB2 ameliorates doxorubicin-induced cardiomyopathy through interaction with NDUFV2 and restoration of mitochondrial complex I function. Redox Biol. 2023;65:102812.
Yan CJ, Gong LL, Chen L, Xu M, Abou-Hamdan H, Tang ML, et al. PHB2 (prohibitin 2) promotes PINK1-PRKN/Parkin-dependent mitophagy by the PARL-PGAM5-PINK1 axis. Autophagy. 2020;16:419–34.
Wai T, Langer T. Mitochondrial dynamics and metabolic regulation. Trends Endocrinol Metab. 2016;27:105–17.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China, including the Regional Innovation and Development Joint Fund (Grant No. U22A20269), the Key Program (Grant No. 81530012), and the Young Scientists Fund (Grant No. 82300270). Additional support was provided by the National Key R&D Program of China (Grant No. 2018YFC1311300).
Author information
Authors and Affiliations
Contributions
DL, JHL and QZT designed research; DL, JHL, YYG, FXX, WYL performed the experiments; DL, YYG, YJC and MZ analyzed data; DL and JHL wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, D., Li, Jh., Guo, Yy. et al. PHB2 protects against pressure overload-induced myocardial remodeling in mice via stabilizing TOMM40 and regulating mitochondrial morphofunctional homeostasis. Acta Pharmacol Sin 46, 3217–3229 (2025). https://doi.org/10.1038/s41401-025-01613-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-025-01613-8