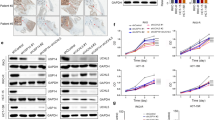
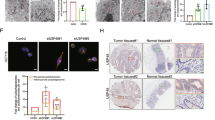
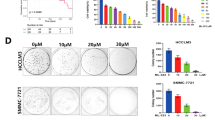
Abstract
Ubiquitin-specific protease 14 (USP14) is a crucial modulator of proteasomal function and cellular proteostasis, which plays an important role in the development and progression of various cancers including colorectal cancer (CRC). In this study we screened 670 covalent compounds using the in vitro Ub-AMC hydrolysis assay, and identified AKOS, initially a Chikungunya virus inhibitor, as a novel small-molecule inhibitor of USP14. We showed that AKOS inhibiting USP14 deubiquitinase activity with an IC50 value of 0.98 μM. AKOS directly bound to USP14, covalently modifying the active-site cysteine residue (Cys114), thereby effectively inhibiting its deubiquitinase activity. We demonstrated that inhibition of USP14 by AKOS might destabilize MEF2D, a critical substrate, resulting in downregulation of the expression and translation of ECM-related transcription factors such as ITGB4. AKOS exhibited potent anti-cancer effects: the USP14 inhibitor significantly inhibited the proliferation and metastasis of CRC cells in vitro with IC50 values of 9.88 and 16.57 μM, respectively, in SW620 cells and HCT116 cells. Intratumoral injection of AKOS (15, 30 mg/kg, every 5 days) effectively suppressed the tumor growth in HCT116 xenograft mouse models in vivo. Collectively, we demonstrate that AKOS is a promising chemical probe for targeting USP14 in CRC, offering a novel strategy for disrupting the malignant progression of CRC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Manasanch EE, Orlowski RZ. Proteasome inhibitors in cancer therapy. Nat Rev Clin Oncol. 2017;14:417–33.
Narayanan S, Cai C, Assaraf YG, Guo H, Cui Q, Wei L, et al. Targeting the ubiquitin-proteasome pathway to overcome anti-cancer drug resistance. Drug Resist Updat. 2020;48:100663.
Curran MP, Mckeage K. Bortezomib: a review of its use in patients with multiple myeloma. Drugs. 2009;69:859–88.
Popovic D, Vucic D, Dikic I. Ubiquitination in disease pathogenesis and treatment. Nat Med. 2014;20:1242–53.
Cockram PE, Kist M, Prakash S, Chen S, Wertz IE, Vucic D. Ubiquitination in the regulation of inflammatory cell death and cancer. Cell Death Differ. 2021;28:591–605.
Harrigan JA, Jacq X, Martin NM, Jackson SP. Deubiquitylating enzymes and drug discovery: emerging opportunities. Nat Rev Drug Discov. 2018;17:57–78.
Dewson G, Eichhorn PJA, Komander D. Deubiquitinases in cancer. Nat Rev Cancer. 2023;23:842–62.
Ren J, Yu P, Liu S, Li R, Niu X, Chen Y, et al. Deubiquitylating enzymes in cancer and immunity. Adv Sci (Weinh). 2023;10:e2303807.
Biller LH, Schrag D. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA. 2021;325:669–85.
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467–80.
Haller DG, Tabernero J, Maroun J, de Braud F, Price T, Van Cutsem E, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29:1465–71.
Adenis A, de la Fouchardiere C, Paule B, Burtin P, Tougeron D, Wallet J, et al. Survival, safety, and prognostic factors for outcome with regorafenib in patients with metastatic colorectal cancer refractory to standard therapies: results from a multicenter study (REBECCA) nested within a compassionate use program. BMC Cancer. 2016;16:412.
Watanabe J, Muro K, Shitara K, Yamazaki K, Shiozawa M, Ohori H, et al. Panitumumab vs bevacizumab added to standard first-line chemotherapy and overall survival among patients with RAS wild-type, left-sided metastatic colorectal cancer: a randomized clinical trial. JAMA. 2023;329:1271–82.
Ganesh K, Stadler ZK, Cercek A, Mendelsohn RB, Shia J, Segal NH, et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol. 2019;16:361–75.
Zhou Y, Wu J, Fu X, Du W, Zhou L, Meng X, et al. OTUB1 promotes metastasis and serves as a marker of poor prognosis in colorectal cancer. Mol Cancer. 2014;13:258.
Zhong J, Zhao M, Ma Y, Luo Q, Liu J, Wang J, et al. UCHL1 acts as a colorectal cancer oncogene via activation of the beta-catenin/TCF pathway through its deubiquitinating activity. Int J Mol Med. 2012;30:430–6.
Trulsson F, Akimov V, Robu M, van Overbeek N, Berrocal DAP, Shah RG, et al. Deubiquitinating enzymes and the proteasome regulate preferential sets of ubiquitin substrates. Nat Commun. 2022;13:2736.
He L, Yu C, Qin S, Zheng E, Liu X, Liu Y, et al. The proteasome component PSMD14 drives myelomagenesis through a histone deubiquitinase activity. Mol Cell. 2023;83:4000–16.
Wang F, Ning S, Yu B, Wang Y. USP14: structure, function, and target inhibition. Front Pharmacol. 2021;12:801328.
Wang D, Ma H, Zhao Y, Zhao J. Ubiquitin-specific protease 14 is a new therapeutic target for the treatment of diseases. J Cell Physiol. 2021;236:3396–405.
Liu B, Chen J, Zhang S. Emerging role of ubiquitin-specific protease 14 in oncogenesis and development of tumor: therapeutic implication. Life Sci. 2019;239:116875.
Shi D, Wu X, Jian Y, Wang J, Huang C, Mo S, et al. USP14 promotes tryptophan metabolism and immune suppression by stabilizing IDO1 in colorectal cancer. Nat Commun. 2022;13:5644.
Du X, Ke S, Liang X, Gao J, Xie X, Qi L, et al. USP14 promotes colorectal cancer progression by targeting JNK for stabilization. Cell Death Dis. 2023;14:56.
Lu B, Sun Y, Chen B, Yang B, He Q, Li J, et al. ZDHHC20-driven s-palmitoylation of CD80 is required for its costimulatory function. Acta Pharmacol Sin. 2024;45:1214–23.
Yuan M, Chen X, Sun Y, Jiang L, Xia Z, Ye K, et al. ZDHHC12-mediated claudin-3 s-palmitoylation determines ovarian cancer progression. Acta Pharm Sin B. 2020;10:1426–39.
Xiang J, Zhang N, Du A, Li J, Luo M, Wang Y, et al. A ubiquitin-dependent switch on MEF2d senses pro-metastatic niche signals to facilitate intrahepatic metastasis of liver cancer. Adv Sci (Weinh). 2023;10:e2305550.
Zhao C, Gong J, Bai Y, Yin T, Zhou M, Pan S, et al. A self-amplifying USP14-TAZ loop drives the progression and liver metastasis of pancreatic ductal adenocarcinoma. Cell Death Differ. 2023;30:1–15.
Xia X, Huang C, Liao Y, Liu Y, He J, Guo Z, et al. Inhibition of USP14 enhances the sensitivity of breast cancer to enzalutamide. J Exp Clin Cancer Res. 2019;38:220.
Lee B, Lee MJ, Park S, Oh D, Elsasser S, Chen P, et al. Enhancement of proteasome activity by a small-molecule inhibitor of USP14. Nature. 2010;467:179–84.
Tian Z, D’Arcy P, Wang X, Ray A, Tai Y, Hu Y, et al. A novel small molecule inhibitor of deubiquitylating enzyme USP14 and UCHL5 induces apoptosis in multiple myeloma and overcomes bortezomib resistance. Blood. 2014;123:706–16.
Xu D, Shan B, Lee B, Zhu K, Zhang T, Sun H, et al. Phosphorylation and activation of ubiquitin-specific protease-14 by akt regulates the ubiquitin-proteasome system. Elife. 2015;4:e10510.
Yuan T, Zeng C, Liu J, Zhao C, Ge F, Li Y, et al. Josephin domain containing 2 (JOSD2) promotes lung cancer by inhibiting LKB1 (liver kinase b1) activity. Signal Transduct Target Ther. 2024;9:11.
Jadav SS, Sinha BN, Hilgenfeld R, Pastorino B, de Lamballerie X, Jayaprakash V. Thiazolidone derivatives as inhibitors of chikungunya virus. Eur J Med Chem. 2015;89:172–8.
Huynh K, Partch CL. Analysis of protein stability and ligand interactions by thermal shift assay. Curr Protoc Protein Sci. 2015;79:28–9.
Abo M, Li C, Weerapana E. Isotopically-labeled iodoacetamide-alkyne probes for quantitative cysteine-reactivity profiling. Mol Pharmacol. 2018;15:743–9.
Xu D, Shan B, Sun H, Xiao J, Zhu K, Xie X, et al. USP14 regulates autophagy by suppressing k63 ubiquitination of beclin 1. Genes Dev. 2016;30:1718–30.
Lv C, Wang S, Lin L, Wang C, Zeng K, Meng Y, et al. USP14 maintains HIF1-alpha stabilization via its deubiquitination activity in hepatocellular carcinoma. Cell Death Dis. 2021;12:803.
Kim HT, Goldberg AL. UBL domain of usp14 and other proteins stimulates proteasome activities and protein degradation in cells. Proc Natl Acad Sci USA. 2018;115:e11642–50.
Chakraborty J, von Stockum S, Marchesan E, Caicci F, Ferrari V, et al. USP14 inhibition corrects an in vivo model of impaired mitophagy. EMBO Mol Med. 2018;10:e9014.
Mialki RK, Zhao J, Wei J, Mallampalli DF, Zhao Y. Overexpression of USP14 protease reduces I-κB protein levels and increases cytokine release in lung epithelial cells. J Biol Chem. 2013;288:15437–41.
Wei J, Dong S, Bowser RK, Khoo A, Zhang L, et al. Regulation of the ubiquitylation and deubiquitylation of CREB-binding protein modulates histone acetylation and lung inflammation. Sci Signal. 2017;10:eaak9660.
Liu B, Jiang S, Li M, Xiong X, Zhu M, et al. Proteome-wide analysis of USP14 substrates revealed its role in hepatosteatosis via stabilization of FASN. Nat Commun. 2018;9:4770.
Boselli M, Lee BH, Robert J, Prado MA, Min SW, et al. An inhibitor of the proteasomal deubiquitinating enzyme USP14 induces tau elimination in cultured neurons. J Biol Chem. 2017;292:19209–25.
Vodenkova S, Buchler T, Cervena K, Veskrnova V, Vodicka P, Vymetalkova V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: past, present and future. Pharmacol Ther. 2020;206:107447.
Ding W, Wang J, Wu J, Liu A, Jiang L, Zhang H, et al. Targeting proteasomal deubiquitinases USP14 and UCHL5 with b-AP15 reduces 5-fluorouracil resistance in colorectal cancer cells. Acta Pharmacol Sin. 2023;44:2537–48.
Wang X, Fang Y, Liang W, Wong CC, Qin H, Gao Y, et al. Fusobacterium nucleatum facilitates anti-PD-1 therapy in microsatellite stable colorectal cancer. Cancer Cell. 2024;42:1729–46.
Liu C, Liu R, Wang B, Lian J, Yao Y, Sun H, et al. Blocking IL-17a enhances tumor response to anti-PD-1 immunotherapy in microsatellite stable colorectal cancer. J Immunother Cancer. 2021;9:e001895.
Sun L, Liu R, Wu Z, Liu Z, Wan AH, Yan S, et al. Galectin-7 induction by EHMT2 inhibition enhances immunity in microsatellite stability colorectal cancer. Gastroenterology. 2024;166:466–82.
Liu D, Li M, Zhao Z, Zhou L, Zhi F, Guo Z, et al. Targeting the TRIM14/USP14 axis enhances immunotherapy efficacy by inducing autophagic degradation of PD-l1. Cancer Res. 2024;84:2806–19.
Acknowledgements
The research was supported by National Natural Science Foundation of China (No. U21A20420 to Bo Yang), Youth Fund of the National Natural Science Foundation of China (No. 82304514 to Li Jiang), Zhejiang Provincial Natural Science Foundation of China (No. LQ23H310004 to Li Jiang), Zhejiang Provincial Natural Science Foundation of China (No. LQ22H310006 to Meng Yuan).
Author information
Authors and Affiliations
Contributions
BL, QJH, JC, LJ, and BY conceived and designed the study. BL, YYS, JHZ, DNC, YG, YLC, CHP, ZYC, and MY performed the experiments. BL and YYS collected and assembled the data. BL and JC wrote the manuscript. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, B., Sun, Yy., Zhou, Jh. et al. Identification of AKOS, a Chikungunya virus inhibitor, as a USP14 inhibitor for colorectal cancer treatment. Acta Pharmacol Sin 46, 3302–3313 (2025). https://doi.org/10.1038/s41401-025-01616-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-025-01616-5