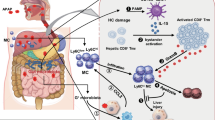
Abstract
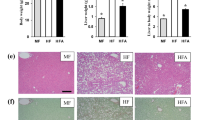
Acetaminophen (APAP)-induced liver injury (AILI) is a leading cause of acute liver failure, with limited preventive or therapeutic options. The role of betaine-homocysteine methyltransferase (BHMT), a key enzyme in the methionine cycle, remains unclear. We found that BHMT, primarily expressed in hepatocytes, showed reduced expression in the liver but elevated serum levels in the APAP-induced liver injury (AILI) mouse model. GalNAc-mediated targeted knockdown of Bhmt in hepatocytes aggravated AILI in mice. Through RNA-seq screening, we found that Bhmt deficiency dramatically suppressed stearoyl-coenzyme A desaturase 1 (SCD1) expression. Knockdown of Scd1 also exacerbated AILI. Mechanistically, Bhmt knockdown decreased the DNA methylation of BACH1 (BTB and CNC homology 1), a transcriptional factor, leading to upregulated BACH1 expression in primary mouse hepatocytes (PMHs) treated with APAP. BACH1 then bound to the enhancer region of Scd1, transcriptionally repressing SCD1. Lipidomic analysis revealed that Bhmt or Scd1 deficiency reduced levels of intracellular unsaturated fatty acids, particularly oleic acid (OA), whereas SCD1 overexpression increased OA levels and decreased lipid peroxides. OA administration alleviated AILI and mitigated the hepatotoxicity associated with Bhmt or Scd1 knockdown. Our findings indicate that BHMT mitigates AILI via the BACH1-SCD1-OA axis, suggesting that BHMT could serve as a preventive target for AILI, while increasing OA intake may offer dietary benefits for patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the main text, supplementary information, and Source data. The Raw RNA sequencing data were deposited in the CNCB GSA database under the accession number PRJCA033967.
References
Andrade RJ, Chalasani N, Björnsson ES, Suzuki A, Kullak-Ublick GA, Watkins PB, et al. Drug-induced liver injury. Nat Rev Dis Prim. 2019;5:58.
Ramachandran A, Jaeschke H. Acetaminophen hepatotoxicity: a mitochondrial perspective. Adv Pharmacol. 2019;85:195–219.
Niu B, Lei X, Xu Q, Ju Y, Xu D, Mao L, et al. Protecting mitochondria via inhibiting VDAC1 oligomerization alleviates ferroptosis in acetaminophen-induced acute liver injury. Cell Biol Toxicol. 2022;38:505–30.
Cai X, Cai H, Wang J, Yang Q, Guan J, Deng J, et al. Molecular pathogenesis of acetaminophen-induced liver injury and its treatment options. J Zhejiang Univ Sci B. 2022;23:265.
Wang H, Wu Y, Tang W. Methionine cycle in nonalcoholic fatty liver disease and its potential applications. Biochem Pharmacol. 2022;200:115033.
Li Z, Wang F, Liang B, Su Y, Sun S, Xia S, et al. Methionine metabolism in chronic liver diseases: an update on molecular mechanism and therapeutic implication. Signal Transduct Target Ther. 2020;5:280.
Miners JO, Drew R, Birkett DJ. Mechanism of action of paracetamol protective agents in mice in vivo. Biochem Pharmacol. 1984;33:2995–3000.
Stramentinoli G, Pezzoli C, Galli-Kienle M. Protective role of S-adenosyl-l-methionine against acetaminophen induced mortality and hepatotoxicity in mice. Biochem Pharmacol. 1979;28:3567–71.
Wang C, Ma C, Gong L, Dai S, Li Y. Preventive and therapeutic role of betaine in liver disease: a review on molecular mechanisms. Eur J Pharmacol. 2021;912:174604.
Park EI, Garrow TA. Interaction between dietary methionine and methyl donor intake on rat liver betaine-homocysteine methyltransferase gene expression and organization of the human gene. J Biol Chem. 1999;274:7816–24.
Zeisel S. Choline, other methyl-donors and epigenetics. Nutrients. 2017;9:445.
Jin B, Gong Z, Yang N, Huang Z, Zeng S, Chen H, et al. Downregulation of betaine homocysteine methyltransferase (BHMT) in hepatocellular carcinoma associates with poor prognosis. Tumour Biol. 2016;37:5911–7.
Ofosu-Boateng M, Shaik F, Choi S, Ekuban FA, Gebreyesus LH, Twum E, et al. High-fat diet induced obesity promotes inflammation, oxidative stress, and hepatotoxicity in female FVB/N mice. Biofactors. 2024;50:572–91.
Avila MA, Berasain C, Torres L, Martín-Duce A, Corrales FJ, Yang H, et al. Reduced mRNA abundance of the main enzymes involved in methionine metabolism in human liver cirrhosis and hepatocellular carcinoma. J Hepatol. 2000;33:907–14.
Ji C, Shinohara M, Kuhlenkamp J, Chan C, Kaplowitz N. Mechanisms of protection by the betaine-homocysteine methyltransferase/betaine system in HepG2 cells and primary mouse hepatocytes. Hepatology. 2007;46:1586–96.
Ji C, Shinohara M, Vance D, Than TA, Ookhtens M, Chan C, et al. Effect of transgenic extrahepatic expression of betaine-homocysteine methyltransferase on alcohol or homocysteine-induced fatty liver. Alcohol Clin Exp Res. 2008;32:1049–58.
Gao J, Shi X, Sun Y, Liu X, Zhang F, Shi C, et al. Deficiency of betaine-homocysteine methyltransferase activates glucose-6-phosphate dehydrogenase (G6PD) by decreasing arginine methylation of G6PD in hepatocellular carcinogenesis. Sci China Life Sci. 2024;67:1648–65.
Hu Z, Lausted C, Yoo H, Yan X, Brightman A, Chen J, et al. Quantitative liver-specific protein fingerprint in blood: a signature for hepatotoxicity. Theranostics. 2014;4:215–28.
Qin S, Zhou Y, Gray L, Kusebauch U, McEvoy L, Antoine DJ, et al. Identification of organ-enriched protein biomarkers of acute liver injury by targeted quantitative proteomics of blood in acetaminophen- and carbon-tetrachloride-treated mouse models and acetaminophen overdose patients. J Proteome Res. 2016;15:3724–40.
Garrow TA. Purification, kinetic properties, and cDNA cloning of mammalian betaine-homocysteine methyltransferase. J Biol Chem. 1996;271:22831–8.
Song J-W, Lam SM, Fan X, Cao W-J, Wang S-Y, Tian H, et al. Omics-driven systems interrogation of metabolic dysregulation in COVID-19 pathogenesis. Cell Metab. 2020;32:188–202.e5.
Lam SM, Zhang C, Wang Z, Ni Z, Zhang S, Yang S, et al. A multi-omics investigation of the composition and function of extracellular vesicles along the temporal trajectory of COVID-19. Nat Metab. 2021;3:909–22.
Gu H, Smith ZD, Bock C, Boyle P, Gnirke A, Meissner A. Preparation of reduced representation bisulfite sequencing libraries for genome-scale DNA methylation profiling. Nat Protoc. 2011;6:468–81.
Hua L, Chen W, Meng Y, Qin M, Yan Z, Yang R, et al. The combination of DNA methylome and transcriptome revealed the intergenerational inheritance on the influence of advanced maternal age. Clin Transl Med. 2022;12:e990.
Li X, Zhi Y, Li J, Lei X, Ju Y, Zhang Y, et al. Single-cell RNA sequencing to reveal non-parenchymal cell heterogeneity and immune network of acetaminophen-induced liver injury in mice. Arch Toxicol. 2023;97:1979–95.
Qin Z-X, Zuo L, Zeng Z, Ma R, Xie W, Zhu X, et al. GalNac-siRNA conjugate delivery technology promotes the treatment of typical chronic liver diseases. Expert Opin Drug Deliv. 2025;22:455–69.
Zhang Y, Liu H, Zhen W, Jiang T, Cui J. Advancement of drugs conjugated with GalNAc in the targeted delivery to hepatocytes based on asialoglycoprotein receptor. Carbohydr Res. 2025;552:109426.
Fontanellas A, Berraondo P, Urigo F, Jericó D, Martini PGV, Pastor F, et al. RNA-based therapies in liver metabolic diseases. Gut. 2025. https://doi.org/10.1136/gutjnl-2023-331742.
ALJohani AM, Syed DN, Ntambi JM. Insights into stearoyl-CoA desaturase-1 regulation of systemic metabolism. Trends Endocrinol Metab. 2017;28:831–42.
Guo Z, Huo X, Li X, Jiang C, Xue L. Advances in regulation and function of stearoyl-CoA desaturase 1 in cancer, from bench to bed 2023. Sci China Life Sci. 2023;66:2773–85.
Xie X, Tian L, Zhao Y, Liu F, Dai S, Gu X, et al. BACH1-induced ferroptosis drives lymphatic metastasis by repressing the biosynthesis of monounsaturated fatty acids. Cell Death Dis. 2023;14:48.
Yoshioka H, Aoyagi Y, Fukuishi N, Gui MY, Jin YR, Li XW, et al. Suppressive effect of kamebakaurin on acetaminophen-induced hepatotoxicity by inhibiting lipid peroxidation and inflammatory response in mice. Pharmacol Rep. 2017;69:903–7.
Jaeschke H, Ramachandran A. Oxidant stress and lipid peroxidation in acetaminophen hepatotoxicity. React Oxyg Species. 2018;5:145–58.
Kagan VE, Mao G, Qu F, Angeli JPF, Doll S, Croix CS, et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13:81–90.
Hou Y, Wei D, Bossila EA, Zhang Z, Li S, Bao J, et al. FABP5 deficiency impaired macrophage inflammation by regulating AMPK/NF-κB signaling pathway. J Immunol. 2022;209:2181–91.
Podszun MC, Chung JY, Ylaya K, Kleiner DE, Hewitt SM, Rotman Y. 4-HNE immunohistochemistry and image analysis for detection of lipid peroxidation in human liver samples using vitamin E treatment in NAFLD as a proof of concept. J Histochem Cytochem. 2020;68:635–43.
Pajares MA, Pérez-Sala D. Betaine homocysteine S-methyltransferase: just a regulator of homocysteine metabolism? Cell Mol Life Sci. 2006;63:2792–803.
Roh T, De U, Lim SK, Kim MK, Choi SM, Lim DS, et al. Detoxifying effect of pyridoxine on acetaminophen-induced hepatotoxicity via suppressing oxidative stress injury. Food Chem Toxicol. 2018;114:11–22.
Pascale RM, Simile MM, Calvisi DF, Feo CF, Feo F. S-adenosylmethionine: from the discovery of its inhibition of tumorigenesis to its use as a therapeutic agent. Cells. 2022;11:409.
Grželj J, Mlinarič-Raščan I, Marko PB, Marovt M, Gmeiner T, Šmid A. Polymorphisms in GNMT and DNMT3b are associated with methotrexate treatment outcome in plaque psoriasis. Biomed Pharmacother. 2021;138:111456.
Ji C. Dissection of endoplasmic reticulum stress signaling in alcoholic and non-alcoholic liver injury. J Gastroenterol Hepatol. 2008;23:S16–24.
Zou Y, Wang YN, Ma H, He ZH, Tang Y, Guo L, et al. SCD1 promotes lipid mobilization in subcutaneous white adipose tissue. J Lipid Res. 2020;61:1589–604.
Dziewulska A, Dobosz AM, Dobrzyn A, Smolinska A, Kolczynska K, Ntambi JM, et al. SCD1 regulates the AMPK/SIRT1 pathway and histone acetylation through changes in adenine nucleotide metabolism in skeletal muscle. J Cell Physiol. 2020;235:1129–40.
Dobosz AM, Janikiewicz J, Krogulec E, Dziewulska A, Ajduk A, Szpila M, et al. Inhibition of stearoyl-CoA desaturase 1 in the mouse impairs pancreatic islet morphogenesis and promotes loss of β-cell identity and α-cell expansion in the mature pancreas. Mol Metab. 2023;67:101659.
Fang R, Yang S, Gu X, Li C, Bi N, Wang HL. Early-life exposure to bisphenol A induces dysregulation of lipid homeostasis by the upregulation of SCD1 in male mice. Environ Pollut. 2022;304:119201.
Zhang N, Wang Y, Zhang J, Liu B, Deng X, Xin S, et al. N-glycosylation of CREBH improves lipid metabolism and attenuates lipotoxicity in NAFLD by modulating PPARα and SCD-1. FASEB J. 2020;34:15338–63.
Ascenzi F, De Vitis C, Maugeri-Saccà M, Napoli C, Ciliberto G, Mancini R. SCD1, autophagy and cancer: implications for therapy. J Exp Clin Cancer Res. 2021;40:265.
Gupta S, Wang L, Kruger WD. Betaine supplementation is less effective than methionine restriction in correcting phenotypes of CBS deficient mice. J Inherit Metab Dis. 2016;39:39–46.
Sun Q, Xing X, Wang H, Wan K, Fan R, Liu C, et al. SCD1 is the critical signaling hub to mediate metabolic diseases: mechanism and the development of its inhibitors. Biomed Pharmacother. 2024;170:115586.
Yin Y, Sichler A, Ecker J, Laschinger M, Liebisch G, Höring M, et al. Gut microbiota promote liver regeneration through hepatic membrane phospholipid biosynthesis. J Hepatol. 2023;78:820–35.
Piccinin E, Cariello M, Santis SD, Ducheix S, Sabbà C, Ntambi JM, et al. Role of oleic acid in the gut-liver axis: from diet to the regulation of its synthesis via stearoyl-CoA desaturase 1 (SCD1). Nutrients. 2019;11:2283.
Bhattacharjee B, Pal PK, Chattopadhyay A, Bandyopadhyay D. Oleic acid protects against cadmium induced cardiac and hepatic tissue injury in male Wistar rats: A mechanistic study. Life Sci. 2020;244:117324.
Nishizawa H, Yamanaka M, Igarashi K. Ferroptosis: regulation by competition between NRF2 and BACH1 and propagation of the death signal. FEBS J. 2023;290:1688–704.
Mann J, Reznik E, Santer M, Fongheiser MA, Smith N, Hirschhorn T, et al. Ferroptosis inhibition by oleic acid mitigates iron-overload-induced injury. Cell Chem Biol. 2024;31:249–64.e7.
Magtanong L, Ko PJ, To M, Cao JY, Forcina GC, Tarangelo A, et al. Exogenous monounsaturated fatty acids promote A ferroptosis-resistant cell state. Cell Chem Biol. 2019;26:420–32.e9.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82370597 to XBL, 32171175 to XBL, 82203500 to CML, and 82260120 to XMY), the General Program of Shanghai Natural Science Foundation (25ZR1401075 to XLZ), the Natural Science Foundation of Ningxia (2023AAC03200 to XMY), the Scientific Research Project of Higher Education of Ningxia (NYG2022043 to XMY), and Tianjin Health Research Project (TJWJ2023MS002 to MYM). We thank Dr. Dan Meng from the School of Basic Medical Sciences, Fudan University, for kindly providing the BACH1 antibody (used for ChIP-qPCR), Ad-BACH1, Ad-shBach1, as well as for her insightful discussions about the project.
Author information
Authors and Affiliations
Contributions
YTZ and XBL designed and conducted the experiments; QSJ, JYC, NBZ, YJ, ZYL, YZ, and YND conducted the experiments; CML, XMY, XLZ, MYM, and YLX analyzed and interpreted the data; YTZ, YMM, and XBL wrote the manuscript; YLX and XBL conceptualized and supervised the study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Yt., Yang, Xm., Jin, Qs. et al. Betaine-homocysteine methyltransferase protects against acetaminophen-induced acute liver failure via BACH1-SCD1-oleic acid axis. Acta Pharmacol Sin 47, 119–134 (2026). https://doi.org/10.1038/s41401-025-01622-7
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-025-01622-7