Abstract
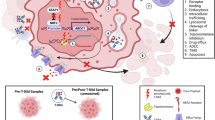
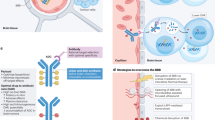
Antibody-drug conjugate (ADC) represents a promising paradigm for tumor-targeted delivery of chemotherapy. Trastuzumab deruxtecan (T-Dxd/DS-8201), a second-generation HER2-ADC, has significantly improved treatment outcomes for breast cancer patients. But due to the large molecular weight, the performance of ADC is still limited by lower tumor penetration, insufficient BBB permeability, and prolonged systemic exposure to normal tissues. In this study, we generated novel anti-HER2 nanobodies (VHH2, VHH3) that exhibited outstanding target affinity and tumor inhibition. After i.v. injection, VHH3-Fc fusion distributed 4 to 5-fold higher in subcutaneous tumor and intracranial tumor compared with trastuzumab. VHH3-Fc and VHH3-ABD were also more penetrant in an in vitro BBB permeability assay. Site-specific conjugation of VHH3-Fc or VHH3-ABD fusions with anti-microtubule MMAE or anti-topoisomerase-1 Dxd payload produced nanobody-drug conjugates (NDCs) with highly potent and durable antitumor efficacy. When evaluated on the same linker-payload (GGFG-Dxd) dosages, VHH3-Fc-Dxd (DAR3.9) outperformed T-Dxd (DAR8) in both the subcutaneous and intracranial tumor models. Moreover, IHC staining and RNA-seq analysis of the treated tumor tissues revealed the involvement of the cGAS-STING-IFNs pathway in mediating the drug activity. Gene expression and protein function were more profoundly modulated by VHH3-Fc-Dxd than T-Dxd. Unlike the higher tumor distribution, the mouse serum PK study revealed a faster clearance (T1/2), reduced exposure (AUC), and higher volume distribution (Vz) for VHH3-Fc-Dxd relative to T-Dxd. Our results provide an example for the next generation HER2-NDC with substantially differentiated pharmacokinetics and pharmacodynamics profiles that will further benefit treatment outcomes and therapeutic windows.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All relevant data are within the manuscript files and are available upon request.
References
Yoon J, Oh DY. HER2-targeted therapies beyond breast cancer - an update. Nature reviews. Clin Oncol. 2024;21:675–700.
Cronin KA, Harlan LC, Dodd KW, Abrams JS, Ballard-Barbash R. Population-based estimate of the prevalence of HER-2 positive breast cancer tumors for early stage patients in the US. Cancer Invest. 2010;28:963–8.
Van Cutsem E, Bang YJ, Feng-Yi F, Xu JM, Lee KW, Jiao SC, et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2015;18:476–84.
Raghav K, Siena S, Takashima A, Kato T, Van den Eynde M, Pietrantonio F, et al. Trastuzumab deruxtecan in patients with HER2-positive advanced colorectal cancer (DESTINY-CRC02): primary results from a multicentre, randomised, phase 2 trial. Lancet Oncol. 2024;25:1147–62.
Gutierrez C, Schiff R. HER2: biology, detection, and clinical implications. Arch Pathol Lab Med. 2011;135:55–62.
Madson JG, Lynch DT, Svoboda J, Ophardt R, Yanagida J, Putta SK, et al. Erbb2 suppresses DNA damage-induced checkpoint activation and UV-induced mouse skin tumorigenesis. Am J Pathol. 2009;174:2357–66.
Boone JJ, Bhosle J, Tilby MJ, Hartley JA, Hochhauser D. Involvement of the HER2 pathway in repair of DNA damage produced by chemotherapeutic agents. Mol Cancer Ther. 2009;8:3015–23.
Garcia-Rendueles MER, Krishnamoorthy G, Saqcena M, Acuna-Ruiz A, Revilla G, de Stanchina E, et al. Yap governs a lineage-specific neuregulin1 pathway-driven adaptive resistance to RAF kinase inhibitors. Mol Cancer. 2022;21:213.
Kodack DP, Askoxylakis V, Ferraro GB, Sheng Q, Badeaux M, Goel S, et al. The brain microenvironment mediates resistance in luminal breast cancer to PI3K inhibition through HER3 activation. Sci Transl Med. 2017;9:eaal4682.
Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer. 2019;121:725–37.
Solanki HS, Welsh EA, Fang B, Izumi V, Darville L, Stone B, et al. Cell type-specific adaptive signaling responses to KRAS(G12C) Inhibition. Clin Cancer Res. 2021;27:2533–48.
Amiri-Kordestani L, Blumenthal GM, Xu QC, Zhang L, Tang SW, Ha L, et al. FDA approval: ado-trastuzumab emtansine for the treatment of patients with HER2-positive metastatic breast cancer. Clin Cancer Res. 2014;20:4436–41.
Hunter FW, Barker HR, Lipert B, Rothe F, Gebhart G, Piccart-Gebhart MJ, et al. Mechanisms of resistance to trastuzumab emtansine (T-DM1) in HER2-positive breast cancer. Br J Cancer. 2020;122:603–12.
Paz-Manrique R, Pinto JA, Gomez Moreno HL. Antibody-drug conjugates in breast cancer: toward a molecular perspective into clinical practice. JCO Precis Oncol. 2024;8:e2400173.
Swain SM, Shastry M, Hamilton E. Targeting HER2-positive breast cancer: advances and future directions. Nat Rev Drug Discov. 2023;22:101–26.
Colombo R, Rich JR. The therapeutic window of antibody drug conjugates: A dogma in need of revision. Cancer Cell. 2022;40:1255–63.
Guidi L, Pellizzari G, Tarantino P, Valenza C, Curigliano G. Resistance to antibody-drug conjugates targeting HER2 in breast cancer: molecular landscape and future challenges. Cancers. 2023;15:1130.
Shitara K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D, et al. Trastuzumab deruxtecan in HER2-positive advanced gastric cancer: exploratory biomarker analysis of the randomized, phase 2 DESTINY-Gastric01 trial. Nat Med. 2024;30:1933–42.
Maadi H, Soheilifar MH, Choi WS, Moshtaghian A, Wang Z. Trastuzumab mechanism of action; 20 years of research to unravel a dilemma. Cancers. 2021;13:3540.
Abuhelwa Z, Alloghbi A, Alqahtani A, Nagasaka M. Trastuzumab Deruxtecan-induced interstitial lung disease/Pneumonitis in ERBB2-positive advanced solid malignancies: a systematic review. Drugs. 2022;82:979–87.
Murrell DH, Foster PJ, Chambers AF. Brain metastases from breast cancer: lessons from experimental magnetic resonance imaging studies and clinical implications. J Mol Med. 2014;92:5–12.
Dasararaju R, Mehta A. Current advances in understanding and managing secondary brain metastasis. CNS Oncol. 2013;2:75–85.
Starkweather CK, Choi BD, Alvarez-Breckenridge C, Brastianos PK, Oh K, Wang N, et al. Initial approach to the patient with multiple newly diagnosed brain metastases. Neurosurg Clin N. Am. 2020;31:505–13.
Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271–7.
Chen PC, Yeh YM, Chu CT, Su PF, Chiu PH, Lin BW, et al. HER2 amplification in colorectal cancer with brain metastasis: A propensity score matching study. Eur J Cancer. 2023;181:62–9.
Lai MY, Guan WL, Yang J, Sun YT, Lu SX, Yang LQ, et al. The relationship between brain metastasis and HER2 expression status in gastric cancer. Clin Transl Oncol. 2024;26:765–73.
Kodack DP, Chung E, Yamashita H, Incio J, Duyverman AM, Song Y, et al. Combined targeting of HER2 and VEGFR2 for effective treatment of HER2-amplified breast cancer brain metastases. Proc Natl Acad Sci USA. 2012;109:E3119–27.
Montemurro F, Delaloge S, Barrios CH, Wuerstlein R, Anton A, Brain E, et al. Trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer and brain metastases: exploratory final analysis of cohort 1 from KAMILLA, a single-arm phase IIIb clinical trial(☆). Ann Oncol. 2020;31:1350–8.
Michelon I, Vilbert M, Marinho AD, Castro CER, Dacoregio MI, Stecca C, et al. Trastuzumab deruxtecan in human epidermal growth factor receptor 2-positive breast cancer brain metastases: a systematic review and meta-analysis. ESMO Open. 2024;9:102233.
Olson EM, Abdel-Rasoul M, Maly J, Wu CS, Lin NU, Shapiro CL. Incidence and risk of central nervous system metastases as site of first recurrence in patients with HER2-positive breast cancer treated with adjuvant trastuzumab. Ann Oncol. 2013;24:1526–33.
Lewis Phillips GD, Nishimura MC, Lacap JA, Kharbanda S, Mai E, Tien J, et al. Trastuzumab uptake and its relation to efficacy in an animal model of HER2-positive breast cancer brain metastasis. Breast Cancer Res Treat. 2017;164:581–91.
Hurvitz SA, Kim SB, Chung WP, Im SA, Park YH, Hegg R, et al. Trastuzumab deruxtecan versus trastuzumab emtansine in HER2-positive metastatic breast cancer patients with brain metastases from the randomized DESTINY-Breast03 trial. ESMO Open. 2024;9:102924.
Ogitani Y, Hagihara K, Oitate M, Naito H, Agatsuma T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016;107:1039–46.
Nessler I, Khera E, Vance S, Kopp A, Qiu Q, Keating TA, et al. Increased tumor penetration of single-domain antibody-drug conjugates improves in vivo efficacy in prostate cancer models. Cancer Res. 2020;80:1268–78.
Bannas P, Lenz A, Kunick V, Fumey W, Rissiek B, Schmid J, et al. Validation of nanobody and antibody based in vivo tumor xenograft NIRF-imaging experiments in mice using ex vivo flow cytometry and microscopy. J Vis Exp 2015;98:e52462.
Rutgers KS, Nabuurs RJ, van den Berg SA, Schenk GJ, Rotman M, Verrips CT, et al. Transmigration of beta amyloid specific heavy chain antibody fragments across the in vitro blood-brain barrier. Neuroscience. 2011;190:37–42.
Li T, Bourgeois JP, Celli S, Glacial F, Le Sourd AM, Mecheri S, et al. Cell-penetrating anti-GFAP VHH and corresponding fluorescent fusion protein VHH-GFP spontaneously cross the blood-brain barrier and specifically recognize astrocytes: application to brain imaging. FASEB J. 2012;26:3969–79.
Jin BK, Odongo S, Radwanska M, Magez S. NANOBODIES®: A review of diagnostic and therapeutic applications. Int J Mol Sci. 2023;24:5994.
Li JY, Perry SR, Muniz-Medina V, Wang X, Wetzel LK, Rebelatto MC, et al. A biparatopic HER2-targeting antibody-drug conjugate induces tumor regression in primary models refractory to or ineligible for HER2-targeted therapy. Cancer Cell. 2016;29:117–29.
Shi D, Mi G, Shen Y, Webster TJ. Glioma-targeted dual functionalized thermosensitive Ferri-liposomes for drug delivery through an in vitro blood-brain barrier. Nanoscale. 2019;11:15057–71.
Zakharova M, Tibbe MP, Koch LS, Le‐The H, Leferink AM, van den Berg A, et al. Transwell‐integrated 2 µm thick transparent polydimethylsiloxane membranes with controlled pore sizes and distribution to model the blood‐brain barrier. Adv Mater Technol. 2021;6:2100138.
Bell A, Wang ZJ, Arbabi-Ghahroudi M, Chang TA, Durocher Y, Trojahn U, et al. Differential tumor-targeting abilities of three single-domain antibody formats. Cancer Lett. 2010;289:81–90.
Sleep D, Cameron J, Evans LR. Albumin as a versatile platform for drug half-life extension. Biochim Biophys Acta. 2013;1830:5526–34.
Chiu ML, Goulet DR, Teplyakov A, Gilliland GL. Antibody structure and function: the basis for engineering therapeutics. Antibodies. 2019;8:55.
Sun D, Luo T, Dong P, Zhang N, Chen J, Zhang S, et al. CD86+/CD206+ tumor-associated macrophages predict prognosis of patients with intrahepatic cholangiocarcinoma. PeerJ. 2020;8:e8458.
Pepin G, Nejad C, Ferrand J, Thomas BJ, Stunden HJ, Sanij E, et al. Topoisomerase 1 inhibition promotes cyclic GMP-AMP Synthase-dependent antiviral responses. mBio. 2017;8:e01611–17.
Marinello J, Arleo A, Russo M, Delcuratolo M, Ciccarelli F, Pommier Y, et al. Topoisomerase I poison-triggered immune gene activation is markedly reduced in human small-cell lung cancers by impairment of the cGAS/STING pathway. Br J Cancer. 2022;127:1214–25.
Wu S, Zhang Q, Zhang F, Meng F, Liu S, Zhou R, et al. HER2 recruits AKT1 to disrupt STING signalling and suppress antiviral defence and antitumour immunity. Nat Cell Biol. 2019;21:1027–40.
Wu Y, Li Q, Kong Y, Wang Z, Lei C, Li J, et al. A highly stable human single-domain antibody-drug conjugate exhibits superior penetration and treatment of solid tumors. Mol Ther. 2022;30:2785–99.
Xu C, Zhu M, Wang Q, Cui J, Huang Y, Huang X, et al. TROP2-directed nanobody-drug conjugate elicited potent antitumor effect in pancreatic cancer. J Nanobiotechnol. 2023;21:410.
Loganzo F, Sung M, Gerber HP. Mechanisms of resistance to antibody-drug conjugates. Mol Cancer Ther. 2016;15:2825–34.
Xia T, Konno H, Ahn J, Barber GN. Deregulation of STING signaling in colorectal carcinoma constrains DNA damage responses and correlates with tumorigenesis. Cell Rep. 2016;14:282–97.
Falahat R, Perez-Villarroel P, Mailloux AW, Zhu G, Pilon-Thomas S, Barber GN, et al. STING signaling in melanoma cells shapes antigenicity and can promote antitumor T-cell activity. Cancer Immunol Res. 2019;7:1837–48.
Oh KS, Nam AR, Bang JH, Jeong Y, Choo SY, Kim HJ, et al. Immunomodulatory effects of trastuzumab deruxtecan through the cGAS-STING pathway in gastric cancer cells. Cell Commun Signal. 2024;22:518.
Alahmari A. Blood-brain barrier overview: structural and functional correlation. Neural Plast. 2021;2021:6564585.
Parodi A, Rudzinska M, Deviatkin AA, Soond SM, Baldin AV, Zamyatnin AA, Jr. Established and emerging strategies for drug delivery across the blood-brain barrier in brain cancer. Pharmaceutics. 2019;11:245.
Acknowledgements
This work was funded by Fudan University (EZF301002), National Natural Science Foundation of China (81373442), NST Major Project of China (2018ZX09711002-008) and NBR 973 Program of China (2013CB932500). The authors thank Animal Experiment Center and Instrument Center at Fudan School of Pharmacy for the technical support.
Author information
Authors and Affiliations
Contributions
YW: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Manuscript Writing. LL: Data curation, Formal analysis, Methodology. QYY: Visualization, Methodology. KY: Conceptualization, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Supervision, Manuscript Writing.
Corresponding author
Ethics declarations
Competing interests
Y. Wang, L. Liu and K. Yu are inventors of a related patent application, international patent application number PCT/CN2023/101129. QY. Yang declares no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Liu, L., Yang, Qy. et al. Novel anti-HER2 nanobody-drug conjugates with enhanced penetration of solid tumor and BBB, reduced systemic exposure and superior antitumor efficacy. Acta Pharmacol Sin 47, 467–480 (2026). https://doi.org/10.1038/s41401-025-01634-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-025-01634-3