Abstract
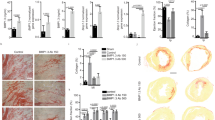
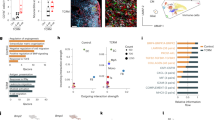
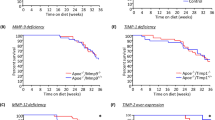
Despite optimized guideline-directed medical therapy, patients with myocardial infarction (MI) often develop heart failure (HF) primarily because of excessive fibrosis. Bone morphogenetic protein 1 (BMP1) plays a critical role in the fibrotic process, yet its specific role in post-MI myocardial fibrosis remains unclear. In this study, we investigated the complex dynamics between BMP1 and fibrotic processes, offering critical insights for novel strategies to mitigate pathological fibrosis in cardiovascular diseases. An experimental MI model was established in mice by ligating the left anterior descending (LAD) coronary artery. We found that the expression levels of BMP1 were significantly elevated in both the serum of MI patients and the cardiac tissues of MI mice. Administration of the BMP1 inhibitor UK383367 (2 mg/kg, i.p., t.i.d., starting the day of myocardial infarction modeling and maintained for 7 days) in MI mice markedly improved cardiac function, reduced myocardial fibrosis, and attenuated the expression of proinflammatory cytokines, including TNF-α, IL-6 and MCP-1. Proteomic profiling revealed that BMP1 was associated with inflammation and oxidative phosphorylation pathways after MI. We demonstrated that UK383367 (250, 500, and 1000 nM) dose-dependently attenuated M1 macrophage polarization, protected mitochondrial function in lipopolysaccharide-stimulated primary macrophages, and inhibited collagen synthesis in Ang II-stimulated cardiac fibroblasts. Overall, these results reveal a pivotal yet detrimental role for BMP1 in driving myocardial fibrosis and amplifying inflammatory cascades after MI. This study highlights the therapeutic potential of the BMP1 inhibitor UK383367 as a promising alternative to conventional antifibrotic strategies, potentially curbing the progression toward HF.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Talman V, Ruskoaho H. Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell Tissue Res. 2016;365:563–81. https://doi.org/10.1007/s00441-016-2431-9.
Nahrendorf M, Pittet MJ, Swirski FK. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation. 2010;121:2437–45. https://doi.org/10.1161/circulationaha.109.916346.
Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res. 2012;110:159–73. https://doi.org/10.1161/circresaha.111.243162.
Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 2014;71:549–74. https://doi.org/10.1007/s00018-013-1349-6.
Kanisicak O, Khalil H, Ivey MJ, Karch J, Maliken BD, Correll RN, et al. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat Commun. 2016;7:12260. https://doi.org/10.1038/ncomms12260.
Humeres C, Frangogiannis NG. Fibroblasts in the infarcted, remodeling, and failing heart. JACC Basic Transl Sci. 2019;4:449–67. https://doi.org/10.1016/j.jacbts.2019.02.006.
Swirski FK, Nahrendorf M. Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat Rev Immunol. 2018;18:733–44. https://doi.org/10.1038/s41577-018-0065-8.
Kubota A, Frangogiannis NG. Macrophages in myocardial infarction. Am J Physiol Cell Physiol. 2022;323:C1304–24. https://doi.org/10.1152/ajpcell.00230.2022.
Peng D, Fu M, Wang M, Wei Y, Wei X. Targeting TGF-β signal transduction for fibrosis and cancer therapy. Mol Cancer. 2022;21:104. https://doi.org/10.1186/s12943-022-01569-x.
Wang Y, Chen Y, Wu J, Shi X. BMP1 promotes keloid by inducing fibroblast inflammation and fibrogenesis. J Cell Biochem. 2024;125:e30609. https://doi.org/10.1002/jcb.30609.
He S, Liu X, Yang Y, Huang W, Xu S, Yang S, et al. Mechanisms of transforming growth factor beta(1)/Smad signalling mediated by mitogen-activated protein kinase pathways in keloid fibroblasts. Br J Dermatol. 2010;162:538–46. https://doi.org/10.1111/j.1365-2133.2009.09511.x.
Massagué J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–810. https://doi.org/10.1101/gad.1350705.
Bai M, Lei J, Wang S, Ding D, Yu X, Guo Y, et al. BMP1 inhibitor UK383,367 attenuates renal fibrosis and inflammation in CKD. Am J Physiol Ren Physiol. 2019;317:F1430–8. https://doi.org/10.1152/ajprenal.00230.2019.
Grgurevic L, Erjavec I, Grgurevic I, Dumic-Cule I, Brkljacic J, Verbanac D, et al. Systemic inhibition of BMP1-3 decreases progression of CCl(4)-induced liver fibrosis in rats. Growth Factors. 2017;35:201–15. https://doi.org/10.1080/08977194.2018.1428966.
Grgurevic L, Macek B, Healy DR, Brault AL, Erjavec I, Cipcic A, et al. Circulating bone morphogenetic protein 1-3 isoform increases renal fibrosis. J Am Soc Nephrol. 2011;22:681–92. https://doi.org/10.1681/asn.2010070722.
Vukicevic S, Colliva A, Kufner V, Martinelli V, Moimas S, Vodret S, et al. Bone morphogenetic protein 1.3 inhibition decreases scar formation and supports cardiomyocyte survival after myocardial infarction. Nat Commun. 2022;13:81. https://doi.org/10.1038/s41467-021-27622-9.
Allan GA, Gedge JI, Nedderman AN, Roffey SJ, Small HF, Webster R. Pharmacokinetics and metabolism of UK-383,367 in rats and dogs: a rationale for long-lived plasma radioactivity. Xenobiotica. 2006;36:399–418. https://doi.org/10.1080/00498250600618177.
Zhang QJ, He Y, Li Y, Shen H, Lin L, Zhu M, et al. Matricellular protein Cilp1 promotes myocardial fibrosis in response to myocardial infarction. Circ Res. 2021;129:1021–35. https://doi.org/10.1161/circresaha.121.319482.
Zacchigna S, Paldino A, Falcão-Pires I, Daskalopoulos EP, Dal Ferro M, Vodret S, et al. Towards standardization of echocardiography for the evaluation of left ventricular function in adult rodents: a position paper of the ESC Working Group on Myocardial Function. Cardiovasc Res. 2021;117:43–59. https://doi.org/10.1093/cvr/cvaa110.
Guo C, Ji W, Yang W, Deng Q, Zheng T, Wang Z, et al. NKRF in cardiac fibroblasts protects against cardiac remodeling post-myocardial infarction via human antigen R. Adv Sci. 2023;10:e2303283. https://doi.org/10.1002/advs.202303283.
Liu Z, Mar KB, Hanners NW, Perelman SS, Kanchwala M, Xing C, et al. A NIK-SIX signalling axis controls inflammation by targeted silencing of non-canonical NF-κB. Nature. 2019;568:249–53. https://doi.org/10.1038/s41586-019-1041-6.
Ma Y, Kuang Y, Bo W, Liang Q, Zhu W, Cai M, et al. Exercise training alleviates cardiac fibrosis through increasing fibroblast growth factor 21 and regulating TGF-β1-Smad2/3-MMP2/9 signaling in mice with myocardial infarction. Int J Mol Sci. 2021;22. https://doi.org/10.3390/ijms222212341.
Ke D, Hong Y, Jiang X, Sun X. Clinical features and vitreous biomarkers of early-onset type 2 diabetes mellitus complicated with proliferative diabetic retinopathy. Diabetes Metab Syndr Obes. 2022;15:1293–303. https://doi.org/10.2147/dmso.S362074.
Tie L, Xiao H, Wu DL, Yang Y, Wang P. A brief guide to good practices in pharmacological experiments: Western blotting. Acta Pharmacol Sin. 2021;42:1015–7. https://doi.org/10.1038/s41401-020-00539-7.
Patel VN, Pineda DL, Berenstein E, Hauser BR, Choi S, Prochazkova M, et al. Loss of Hs3st3a1 or Hs3st3b1 enzymes alters heparan sulfate to reduce epithelial morphogenesis and adult salivary gland function. Matrix Biol. 2021;103-104:37–57. https://doi.org/10.1016/j.matbio.2021.10.002.
Hao H, Cao L, Jiang C, Che Y, Zhang S, Takahashi S, et al. Farnesoid X receptor regulation of the NLRP3 inflammasome underlies cholestasis-associated sepsis. Cell Metab. 2017;25:856–67.e5. https://doi.org/10.1016/j.cmet.2017.03.007.
Zhang X, Zuo X, Yang B, Li Z, Xue Y, Zhou Y, et al. MicroRNA directly enhances mitochondrial translation during muscle differentiation. Cell. 2014;158:607–19. https://doi.org/10.1016/j.cell.2014.05.047.
Sun J, Zhou C, Zhao Y, Zhang X, Chen W, Zhou Q, et al. Quiescin sulfhydryl oxidase 1 promotes sorafenib-induced ferroptosis in hepatocellular carcinoma by driving EGFR endosomal trafficking and inhibiting NRF2 activation. Redox Biol. 2021;41:101942. https://doi.org/10.1016/j.redox.2021.101942.
Foote K, Reinhold J, Yu EPK, Figg NL, Finigan A, Murphy MP, et al. Restoring mitochondrial DNA copy number preserves mitochondrial function and delays vascular aging in mice. Aging Cell. 2018;17:e12773. https://doi.org/10.1111/acel.12773.
Liu Q, Wang X, Hu Y, Zhao JN, Huang CH, Li T, et al. Acetylated tau exacerbates learning and memory impairment by disturbing with mitochondrial homeostasis. Redox Biol. 2023;62:102697. https://doi.org/10.1016/j.redox.2023.102697.
Kim Y, Nurakhayev S, Nurkesh A, Zharkinbekov Z, Saparov A. Macrophage polarization in cardiac tissue repair following myocardial infarction. Int J Mol Sci. 2021;22. https://doi.org/10.3390/ijms22052715.
Zhang F, Zhang L, Qi Y, Xu H. Mitochondrial cAMP signaling. Cell Mol Life Sci. 2016;73:4577–90. https://doi.org/10.1007/s00018-016-2282-2.
Xia PP, Zhang F, Chen C, Wang ZH, Wang N, Li LY, et al. Rac1 relieves neuronal injury induced by oxygenglucose deprivation and re-oxygenation via regulation of mitochondrial biogenesis and function. Neural Regen Res. 2020;15:1937–46. https://doi.org/10.4103/1673-5374.280325.
Witherel CE, Sao K, Brisson BK, Han B, Volk SW, Petrie RJ, et al. Regulation of extracellular matrix assembly and structure by hybrid M1/M2 macrophages. Biomaterials. 2021;269:120667. https://doi.org/10.1016/j.biomaterials.2021.120667.
Baicu CF, Zhang Y, Van Laer AO, Renaud L, Zile MR, Bradshaw AD. Effects of the absence of procollagen C-endopeptidase enhancer-2 on myocardial collagen accumulation in chronic pressure overload. Am J Physiol Heart Circ Physiol. 2012;303:H234–40. https://doi.org/10.1152/ajpheart.00227.2012.
Shen S, He F, Cheng C, Xu B, Sheng J. Uric acid aggravates myocardial ischemia-reperfusion injury via ROS/NLRP3 pyroptosis pathway. Biomed Pharmacother. 2021;133:110990. https://doi.org/10.1016/j.biopha.2020.110990.
Zhang Q, Wang L, Wang S, Cheng H, Xu L, Pei G, et al. Signaling pathways and targeted therapy for myocardial infarction. Signal Transduct Target Ther. 2022;7:78. https://doi.org/10.1038/s41392-022-00925-z.
Bajpai G, Bredemeyer A, Li W, Zaitsev K, Koenig AL, Lokshina I, et al. Tissue resident CCR2- and CCR2+ cardiac macrophages differentially orchestrate monocyte recruitment and fate specification following myocardial injury. Circ Res. 2019;124:263–78. https://doi.org/10.1161/circresaha.118.314028.
Zhuang L, Zong X, Yang Q, Fan Q, Tao R. Interleukin-34-NF-κB signaling aggravates myocardial ischemic/reperfusion injury by facilitating macrophage recruitment and polarization. EBioMedicine. 2023;95:104744. https://doi.org/10.1016/j.ebiom.2023.104744.
Fan Q, Tao R, Zhang H, Xie H, Lu L, Wang T, et al. Dectin-1 contributes to myocardial ischemia/reperfusion injury by regulating macrophage polarization and neutrophil infiltration. Circulation. 2019;139:663–78. https://doi.org/10.1161/circulationaha.118.036044.
Yu H, Zhang F, Yan P, Zhang S, Lou Y, Geng Z, et al. LARP7 protects against heart failure by enhancing mitochondrial biogenesis. Circulation. 2021;143:2007–22. https://doi.org/10.1161/circulationaha.120.050812.
Lin ZJ, Dong X, He H, Jiang JL, Guan ZJ, Li X, et al. A simplified herbal decoction attenuates myocardial infarction by regulating macrophage metabolic reprogramming and phenotypic differentiation via modulation of the HIF-1α/PDK1 axis. Chin Med. 2024;19:75. https://doi.org/10.1186/s13020-024-00933-x.
Zhao H, Liu YJ, Liu ZR, Tang DD, Chen XW, Chen YH, et al. Role of mitochondrial dysfunction in renal fibrosis promoted by hypochlorite-modified albumin in a remnant kidney model and protective effects of antioxidant peptide SS-31. Eur J Pharmacol. 2017;804:57–67. https://doi.org/10.1016/j.ejphar.2017.03.037.
Lin LC, Tu B, Song K, Liu ZY, Sun H, Zhou Y, et al. Mitochondrial quality control in cardiac fibrosis: epigenetic mechanisms and therapeutic strategies. Metabolism. 2023;145:155626. https://doi.org/10.1016/j.metabol.2023.155626.
Xu S, Tao H, Cao W, Cao L, Lin Y, Zhao SM, et al. Ketogenic diets inhibit mitochondrial biogenesis and induce cardiac fibrosis. Signal Transduct Target Ther. 2021;6:54. https://doi.org/10.1038/s41392-020-00411-4.
Acknowledgements
This research was funded by a grant from the Shandong Provincial Natural Science Foundation (ZR2021MH361). The graphical abstract was drawn using FigDraw.
Author information
Authors and Affiliations
Contributions
CHG and QQW designed the study, performed the experiments, and wrote the main manuscript text. JQL performed the experiments and analyzed the data. WJ contributed to the proteomic profiling. LC and MLC provided critical insights and revisions. LYM conducted the statistical analyses. MN and XLL supervised the project and reviewed the final manuscript. All the authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, Ch., Wang, Qq., Li, Jq. et al. BMP1 inhibitor UK383367 improves MI-induced cardiac remodeling and fibrosis in mice via ameliorating macrophage polarization and mitochondrial dysfunction. Acta Pharmacol Sin 47, 315–327 (2026). https://doi.org/10.1038/s41401-025-01655-y
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-025-01655-y