Abstract
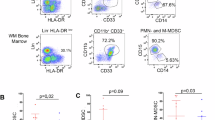
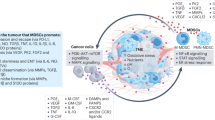
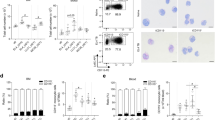
Myeloid-derived suppressor cells (MDSCs) are a category of immature myeloid cells that have an important function in suppressing immune responses in a variety of pathological settings. Thus, MDSCs are the subject of intensive studies regarding their recruitment, expulsion, deactivation, and maturation promotion. Tumor necrosis factor superfamily member 15 (TNFSF15) is produced largely by vascular endothelial cells in mature blood vessels with expression also observed in tumor-associated macrophages (TAMs) and dendritic cells (DCs) within the tumor stroma. In addition to inhibiting the proliferation of vascular endothelial cells and the differentiation of bone marrow-derived endothelial cell progenitors, TNFSF15 is able to promote the maturation of DC, as well as to modulate the polarization of naive M2-macrophages into M1-macrophages capable of eliminating cancer cells, and activate T-cell. In this study, we investigated whether a recombinant TNFSF15 results in a substantial reduction of MDSC accumulation in Lewis lung cancer (LLC) tumor-bearing mice. LLC allograft model mice were administered recombinant TNFSF15 (5 mg·kg−1·d−1, i.p.) for 7 consecutive days. The tumor, bone marrow and spleen were retrieved on Day 8 and analyzed using flow cytometry or immunofluorescence staining. We showed that TNFSF15 treatment significantly inhibited the tumor growth, and caused a substantial reduction of MDSC accumulation in the tumors. The proportions of MDSC in the bone marrows and the spleens were also reduced. The diminished MDSC was mainly the monocyte-like MDSC (M-MDSC) subtype. Additionally, the reduction in M-MDSC population was accompanied by an increase of the proportions of macrophages and DCs in the tumors. We demonstrated that TNFSF15 promoted M-MDSC differentiation by activating the JAK1/STAT3 signaling pathway. Moreover, the treatment gave rise to a markedly escalated accumulation of cytotoxic T cells in the tumors, attributing to tumor growth inhibition. Our results support the view that TNFSF15-driven differentiation of M-MDSC into DCs and macrophages, and the subsequent activation of T cells, may contribute partially to reinstitution of immunity in the tumor microenvironment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Veglia F, Sanseviero E, Gabrilovich DI. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat Rev Immunol. 2021;21:485–98.
Groth C, Hu X, Weber R, Fleming V, Altevogt P, Utikal J, et al. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br J Cancer. 2019;120:16–25.
Li K, Shi H, Zhang B, Ou X, Ma Q, Chen Y, et al. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct Target Ther. 2021;6:362.
Mao Y, Eissler N, Blanc KL, Johnsen JI, Kogner P, Kiessling R. Targeting suppressive myeloid cells potentiates checkpoint inhibitors to control spontaneous neuroblastoma. Clin Cancer Res. 2016;22:3849–59.
Alizadeh D, Trad M, Hanke NT, Larmonier CB, Janikashvili N, Bonnotte B, et al. Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer. Cancer Res. 2014;74:104–18.
Nagaraj S, Youn JI, Weber H, Iclozan C, Lu L, Cotter MJ, et al. Anti-inflammatory triterpenoid blocks immune suppressive function of MDSCs and improves immune response in cancer. Clin Cancer Res. 2010;16:1812–23.
Kusmartsev S, Cheng F, Yu B, Nefedova Y, Sotomayor E, Lush R, et al. All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res. 2003;63:4441–9.
Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013;13:739–52.
Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68.
Nakamura K, Smyth MJ. Myeloid immunosuppression and immune checkpoints in the tumor microenvironment. Cell Mol Immunol. 2020;17:1–12.
Ben-Meir K, Twaik N, Baniyash M. Plasticity and biological diversity of myeloid derived suppressor cells. Curr Opin Immunol. 2018;51:154–61.
Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016;37:208–20.
Ferrer G, Jung B, Chiu PY, Aslam R, Palacios F, Mazzarello AN, et al. Myeloid-derived suppressor cell subtypes differentially influence T-cell function, T-helper subset differentiation, and clinical course in CLL. Leukemia. 2021;35:3163–75.
Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. 2018;19:108–19.
Holtzhausen A, Harris W, Ubil E, Hunter DM, Zhao J, Zhang Y, et al. TAM family receptor kinase inhibition reverses MDSC-mediated suppression and augments anti-PD-1 therapy in melanoma. Cancer Immunol Res. 2019;7:1672–86.
Kumar V, Cheng P, Condamine T, Mony S, Languino LR, McCaffrey JC, et al. CD45 phosphatase inhibits STAT3 transcription factor activity in myeloid cells and promotes tumor-associated macrophage differentiation. Immunity. 2016;44:303–15.
Bitsch R, Kurzay A, Özbay Kurt F, De La Torre C, Lasser S, Lepper A, et al. STAT3 inhibitor napabucasin abrogates MDSC immunosuppressive capacity and prolongs survival of melanoma-bearing mice. J Immunother Cancer. 2022;10:e004384.
Zhai Y, Ni J, Jiang GW, Lu J, Xing L, Lincoln C, et al. VEGI, a novel cytokine of the tumor necrosis factor family, is an angiogenesis inhibitor that suppresses the growth of colon carcinomas in vivo. FASEB J. 1999;13:181–9.
Migone TS, Zhang J, Luo X, Zhuang L, Chen C, Hu B, et al. TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity. 2002;16:479–92.
Xu WD, Li R, Huang AF. Role of TL1A in inflammatory autoimmune diseases: a comprehensive review. Front Immunol. 2022;13:891328.
Hedl M, Abraham C. A TNFSF15 disease-risk polymorphism increases pattern-recognition receptor-induced signaling through caspase-8-induced IL-1. Proc Natl Acad Sci USA. 2014;111:13451–6.
Al-Lamki RS, Wang J, Pober JS, Bradley JR. Co-expression and functional interactions of death receptor 3 and E-selectin in clear cell renal cell carcinoma. Am J Pathol. 2022;192:722–36.
Jiang M, Zhang W, Zhang R, Liu P, Ye Y, Yu W, et al. Cancer exosome-derived miR-9 and miR-181a promote the development of early-stage MDSCs via interfering with SOCS3 and PIAS3 respectively in breast cancer. Oncogene. 2020;39:4681–94.
Ma C, Huang J, Zheng Y, Na Y, Wei J, Shan J, et al. Anti-TL1A monoclonal antibody modulates the dysregulation of Th1/Th17 cells and attenuates granuloma formation in sarcoidosis by inhibiting the PI3K/AKT signaling pathway. Int Immunopharmacol. 2024;137:112360.
Zhao CC, Han QJ, Ying HY, Gu XX, Yang N, Li LY, et al. TNFSF15 facilitates differentiation and polarization of macrophages toward M1 phenotype to inhibit tumor growth. Oncoimmunology. 2022;11:2032918.
Sethi G, Sung B, Aggarwal BB. Therapeutic potential of VEGI/TL1A in autoimmunity and cancer. Adv Exp Med Biol. 2009;647:207–15.
Tian F, Liang PH, Li LY. Inhibition of endothelial progenitor cell differentiation by VEGI. Blood. 2009;113:5352–60.
Liang PH, Tian F, Lu Y, Duan B, Stolz DB, Li LY. Vascular endothelial growth inhibitor (VEGI; TNFSF15) inhibits bone marrow-derived endothelial progenitor cell incorporation into Lewis lung carcinoma tumors. Angiogenesis. 2011;14:61–8.
Tian F, Grimaldo S, Fujita M, Cutts J, Vujanovic NL, Li LY. The endothelial cell-produced antiangiogenic cytokine vascular endothelial growth inhibitor induces dendritic cell maturation. J Immunol. 2007;179:3742–51.
Qi JW, Qin TT, Xu LX, Zhang K, Yang GL, Li J, et al. TNFSF15 inhibits vasculogenesis by regulating relative levels of membrane-bound and soluble isoforms of VEGF receptor 1. Proc Natl Acad Sci USA. 2013;110:13863–8.
Hou W, Medynski D, Wu S, Lin X, Li LY. VEGI-192, a new isoform of TNFSF15, specifically eliminates tumor vascular endothelial cells and suppresses tumor growth. Clin Cancer Res. 2005;11:5595–602.
Bronte V, Wang M, Overwijk WW, Surman DR, Pericle F, Rosenberg SA, et al. Apoptotic death of CD8+ T lymphocytes after immunization: induction of a suppressive population of Mac-1+/Gr-1+ cells. J Immunol. 1998;161:5313–20.
Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74.
Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68:5439–49.
Sade-Feldman M, Kanterman J, Ish-Shalom E, Elnekave M, Horwitz E, Baniyash M. Tumor necrosis factor-α blocks differentiation and enhances suppressive activity of immature myeloid cells during chronic inflammation. Immunity. 2013;38:541–54.
Rodríguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol Rev. 2008;222:180–91.
Awad M, Sen’kova A, Zenkova M, Markov O. The impact of cytokines and tumour-conditioned medium on the properties of murine in vitro generated myeloid-derived suppressor cells. Scand J Immunol. 2025;101:e70001.
Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–11.
Li BH, Garstka MA, Li ZF. Chemokines and their receptors promoting the recruitment of myeloid-derived suppressor cells into the tumor. Mol Immunol. 2020;117:201–15.
Bronte V, Pittet MJ. The spleen in local and systemic regulation of immunity. Immunity. 2013;39:806–18.
Hou Y, Liang HL, Yu X, Liu Z, Cao X, Rao E, et al. Radiotherapy and immunotherapy converge on elimination of tumor-promoting erythroid progenitor cells through adaptive immunity. Sci Transl Med. 2021;13:eabb0130.
Kaczanowska S, Beury DW, Gopalan V, Tycko AK, Qin H, Clements ME, et al. Genetically engineered myeloid cells rebalance the core immune suppression program in metastasis. Cell. 2021;184:2033–52.e21.
Yu J, Green MD, Li S, Sun Y, Journey SN, Choi JE, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med. 2021;27:152–64.
Liu M, Wu C, Luo S, Hua Q, Chen HT, Weng Y, et al. PERK reprograms hematopoietic progenitor cells to direct tumor-promoting myelopoiesis in the spleen. J Exp Med. 2022;219:e20211498.
Pan PY, Ma G, Weber KJ, Ozao-Choy J, Wang G, Yin B, et al. Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer. Cancer Res. 2010;70:99–108.
Huang J, Jochems C, Talaie T, Anderson A, Jales A, Tsang KY, et al. Elevated serum soluble CD40 ligand in cancer patients may play an immunosuppressive role. Blood. 2012;120:3030–8.
Sinha P, Chornoguz O, Clements VK, Artemenko KA, Zubarev RA, Ostrand-Rosenberg S. Myeloid-derived suppressor cells express the death receptor Fas and apoptose in response to T cell-expressed FasL. Blood. 2011;117:5381–90.
He Z, Wang S, Wu J, Xie Y, Li B. Lower expression of TWEAK is associated with poor survival and dysregulate TIICs in lung adenocarcinoma. Dis markers. 2022;2022:8661423.
Hoffmann SHL, Reck DI, Maurer A, Fehrenbacher B, Sceneay JE, Poxleitner M, et al. Visualization and quantification of in vivo homing kinetics of myeloid-derived suppressor cells in primary and metastatic cancer. Theranostics. 2019;9:5869–85.
Trikha P, Carson WE 3rd. Signaling pathways involved in MDSC regulation. Biochim Biophys Acta. 2014;1846:55–65.
Gabrilovich DI. Myeloid-derived suppressor cells. Cancer Immunol Res. 2017;5:3–8.
Mahanti K, Saha J, Sarkar D, Pramanik A, Roy Chattopadhyay N, Bhattacharyya S. Alteration of functionality and differentiation directed by changing gene expression patterns in myeloid-derived suppressor cells (MDSCs) in tumor microenvironment and bone marrow through early to terminal phase of tumor progression. J Leukoc Biol. 2024;115:958–84.
Zhao Y, Du J, Shen X. Targeting myeloid-derived suppressor cells in tumor immunotherapy: current, future and beyond. Front Immunol. 2023;14:1157537.
Acknowledgements
This study was supported in part by National Natural Science Foundation of China (82073064 and 81874167 to LYL; 82473963 to ZSZ), Haihe Laboratory of Cell Ecosystem Innovation Fund (22HHXBSS00020 to LYL), Ministry of Education 111 Project (B20016 to LYL), the Key Projects of Tianjin Science and Technology (24ZXZSSS00150 to ZSZ), and Key Laboratory of Immune Microenvironment and Disease (Ministry of Education) (116019-KJ01000601 to ZSZ).
Author information
Authors and Affiliations
Contributions
YPZ performed most of the cellular, biochemical protein purification, and animal experiments. JS and XYD contributed to the cellular and animal experiments. YPZ and JS analyzed data; XYC, YYW, QJH, and JYW contributed to data analysis and chart analysis production. YPZ and JYW wrote the original manuscript. LYL and ZSZ initiated the project, led the project team, designed the experiment plans, analyzed the results.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, Yp., Sun, J., Cao, Xy. et al. TNFSF15 alleviates myeloid-derived suppressor cell-mediated cancer immunosuppression in mice. Acta Pharmacol Sin 47, 493–503 (2026). https://doi.org/10.1038/s41401-025-01663-y
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-025-01663-y