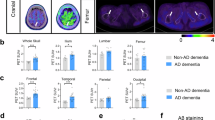
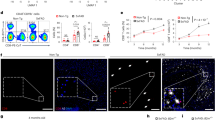
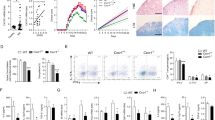
Abstract
Although most AD-related pathological studies are limited to the brain, increasing evidence has demonstrated the contribution of peripheral immune cells to the pathogenesis of AD. We recently demonstrated that meningeal B cells inhibit β-amyloid (Aβ) production in the frontal cortex of young 5×FAD mice. In this study, we explored the precise origin of meningeal B cells. We observed that the AD-like pathology in 5×FAD mice was exacerbated when the germinal center in the lung lymph nodes was specifically destroyed via the intratracheal instillation of anti-CD40 antibodies, whereas it was alleviated via the intratracheal instillation of AAV-mBAFF to overexpress B-cell activating factor in the lungs. We demonstrated that Aβ was drained from the brain via meningeal lymphatics and eventually traveled to the lungs, where it activated B cells via the TLR4/NF-ĸB signaling pathway, whereas the CXCL12-CXCR4 axis regulated lung B-cell infiltration into the frontal cortex. We revealed that the increased number of B cells in the lungs of 5×FAD mice mainly included memory B (Bmem) cells. The supplementation of lung Bmem cells mitigated AD-like pathology in B-cell-deficient μMT-/-/5×FAD mice, which was abolished by using a CXCR4 antagonist. The suppression of CXCL12 expression in frontal microglia via AAV-siCXCL12 inhibited the infiltration of CXCR4+ Bmem cells and increased the Aβ burden in the frontal cortex of 5×FAD mice. Collectively, our results demonstrate an unexpected protective effect of lung Bmem cells on AD-like pathology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Long JM, Holtzman DM. Alzheimer disease: an update on pathobiology and treatment strategies. Cell. 2019;179:312–39.
Chen X, Holtzman DM. Emerging roles of innate and adaptive immunity in Alzheimer’s disease. Immunity. 2022;55:2236–54.
Huang LT, Zhang CP, Wang YB, Wang JH. Association of peripheral blood cell profile with Alzheimer’s disease: a meta-analysis. Front Aging Neurosci. 2022;14:888946.
Song L, Yang YT, Guo Q, Consortium ZIB, Zhao XM. Cellular transcriptional alterations of peripheral blood in Alzheimer’s disease. BMC Med. 2022;20:266.
Bulati M, Buffa S, Martorana A, Gervasi F, Camarda C, Azzarello DM, et al. Double negative (IgG+IgD-CD27-) B cells are increased in a cohort of moderate-severe Alzheimer’s disease patients and show a pro-inflammatory trafficking receptor phenotype. J Alzheimers Dis. 2015;44:1241–51.
Kim K, Wang X, Ragonnaud E, Bodogai M, Illouz T, DeLuca M, et al. Therapeutic B-cell depletion reverses progression of Alzheimer’s disease. Nat Commun. 2021;12:2185.
Feng W, Zhang Y, Ding S, Chen S, Wang T, Wang Z, et al. B lymphocytes ameliorate Alzheimer’s disease-like neuropathology via interleukin-35. Brain Behav Immun. 2023;108:16–31.
Fitzpatrick Z, Frazer G, Ferro A, Clare S, Bouladoux N, Ferdinand J, et al. Gut-educated IgA plasma cells defend the meningeal venous sinuses. Nature. 2020;587:472–6.
Brioschi S, Wang WL, Peng V, Wang M, Shchukina I, Greenberg ZJ, et al. Heterogeneity of meningeal B cells reveals a lymphopoietic niche at the CNS borders. Science. 2021;373:eabf9277.
Janssens R, Struyf S, Proost P. Pathological roles of the homeostatic chemokine CXCL12. Cytokine Growth Factor Rev. 2018;44:51–68.
Schropp V, Chunder R, Dietel B, Tacke S, Kuerten S. The presence of cerebellar B cell aggregates is associated with a specific chemokine profile in the cerebrospinal fluid in a mouse model of multiple sclerosis. J Neuroinflammation. 2023;20:18.
Lee Y, Choi Y, Park EJ, Kwon S, Kim H, Lee JY, et al. Improvement of glymphatic-lymphatic drainage of beta-amyloid by focused ultrasound in Alzheimer’s disease model. Sci Rep. 2020;10:16144.
Chuang JZ, Yang N, Nakajima N, Otsu W, Fu C, Yang HH, et al. Retinal pigment epithelium-specific CLIC4 mutant is a mouse model of dry age-related macular degeneration. Nat Commun. 2022;13:374.
Wang H, Leigh J. Effects of nitric oxide synthase inhibitor omega-nitro-L-arginine methyl ester, on silica-induced inflammatory reaction and apoptosis. Part Fibre Toxicol. 2006;3:14.
Li Q, Chen Y, Feng W, Cai J, Gao J, Ge F, et al. Drainage of senescent astrocytes from brain via meningeal lymphatic routes. Brain Behav Immun. 2022;103:85–96.
Smyth LCD, Xu D, Okar SV, Dykstra T, Rustenhoven J, Papadopoulos Z, et al. Identification of direct connections between the dura and the brain. Nature. 2024;627:165–73.
Schalbetter SM, von Arx AS, Cruz-Ochoa N, Dawson K, Ivanov A, Mueller FS, et al. Adolescence is a sensitive period for prefrontal microglia to act on cognitive development. Sci Adv. 2022;8:eabi6672.
Yardeni T, Eckhaus M, Morris HD, Huizing M, Hoogstraten-Miller S. Retro-orbital injections in mice. Lab Anim. 2011;40:155–60.
Yang Z, Wang W, Zhao L, Wang X, Gimple RC, Xu L, et al. Plasma cells shape the mesenchymal identity of ovarian cancers through transfer of exosome-derived microRNAs. Sci Adv. 2021;7:eabb0737.
Wei SY, Wu TT, Huang JQ, Kang ZP, Wang MX, Zhong YB, et al. Curcumin alleviates experimental colitis via a potential mechanism involving memory B cells and Bcl-6-Syk-BLNK signaling. World J Gastroenterol. 2022;28:5865–80.
Wang QT, Wu YJ, Huang B, Ma YK, Song SS, Zhang LL, et al. Etanercept attenuates collagen-induced arthritis by modulating the association between BAFFR expression and the production of splenic memory B cells. Pharmacol Res. 2013;68:38–45.
Sarvaria A, Madrigal JA, Saudemont A. B cell regulation in cancer and anti-tumor immunity. Cell Mol Immunol. 2017;14:662–74.
Min S, Tao W, Miao Y, Li Y, Wu T, He X, et al. Dual delivery of tetramethylpyrazine and miR-194-5p using soft mesoporous organosilica nanoparticles for acute lung injury therapy. Int J Nanomed. 2023;18:6469–86.
Holtmaat A, Bonhoeffer T, Chow DK, Chuckowree J, De Paola V, Hofer SB, et al. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat Protoc. 2009;4:1128–44.
Tong L, Han SS, Xue Y, Chen MG, Chen FY, Ke W, et al. Single cell optogenetic stimulation by two-photon excitation fluorescence transfer. iScience. 2023;26:107857.
Mestre H, Hablitz LM, Xavier AL, Feng W, Zou W, Pu T, et al. Aquaporin-4-dependent glymphatic solute transport in the rodent brain. Elife. 2018;7:e40070.
Weber DJ, Gracon AS, Ripsch MS, Fisher AJ, Cheon BM, Pandya PH, et al. The HMGB1-RAGE axis mediates traumatic brain injury-induced pulmonary dysfunction in lung transplantation. Sci Transl Med. 2014;6:252ra124.
Mumaw CL, Levesque S, McGraw C, Robertson S, Lucas S, Stafflinger JE, et al. Microglial priming through the lung-brain axis: the role of air pollution-induced circulating factors. FASEB J. 2016;30:1880–91.
Gregoire C, Spinelli L, Villazala-Merino S, Gil L, Holgado MP, Moussa M, et al. Viral infection engenders bona fide and bystander subsets of lung-resident memory B cells through a permissive mechanism. Immunity. 2022;55:1216–33.e9.
Mackay F, Browning JL. BAFF: a fundamental survival factor for B cells. Nat Rev Immunol. 2002;2:465–75.
Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006;26:10129–40.
Locci A, Orellana H, Rodriguez G, Gottliebson M, McClarty B, Dominguez S, et al. Comparison of memory, affective behavior, and neuropathology in APP(NLGF) knock-in mice to 5xFAD and APP/PS1 mice. Behav Brain Res. 2021;404:113192.
Kosel F, Torres Munoz P, Yang JR, Wong AA, Franklin TB. Age-related changes in social behaviours in the 5xFAD mouse model of Alzheimer’s disease. Behav Brain Res. 2019;362:160–72.
Da Mesquita S, Louveau A, Vaccari A, Smirnov I, Cornelison RC, Kingsmore KM, et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature. 2018;560:185–91.
Louveau A, Herz J, Alme MN, Salvador AF, Dong MQ, Viar KE, et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat Neurosci. 2018;21:1380–91.
Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4:147ra111.
Balczon R, Lin MT, Voth S, Nelson AR, Schupp JC, Wagener BM, et al. Lung endothelium, tau, and amyloids in health and disease. Physiol Rev. 2024;104:533–87.
Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–61.
Hua Z, Hou B. TLR signaling in B-cell development and activation. Cell Mol Immunol. 2013;10:103–6.
Lemarquis AL, Einarsdottir HK, Kristjansdottir RN, Jonsdottir I, Ludviksson BR. Transitional B Cells and TLR9 Responses Are Defective in Selective IgA Deficiency. Front Immunol. 2018;9:909.
Shanehbandi D, Majidi J, Kazemi T, Baradaran B, Aghebati-Maleki L. CD20-based immunotherapy of B-cell derived hematologic malignancies. Curr Cancer Drug Targets. 2017;17:423–44.
Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–41.
Busse M, Michler E, von Hoff F, Dobrowolny H, Hartig R, Frodl T, et al. Alterations in the peripheral immune system in dementia. J Alzheimers Dis. 2017;58:1303–13.
Escott-Price V, Bellenguez C, Wang LS, Choi SH, Harold D, Jones L, et al. Gene-wide analysis detects two new susceptibility genes for Alzheimer’s disease. PLoS ONE. 2014;9:e94661.
Shen P, Fillatreau S. Antibody-independent functions of B cells: a focus on cytokines. Nat Rev Immunol. 2015;15:441–51.
Lefrancais E, Ortiz-Munoz G, Caudrillier A, Mallavia B, Liu F, Sayah DM, et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature. 2017;544:105–9.
Wilk MM, Misiak A, McManus RM, Allen AC, Lynch MA, Mills KHG. Lung CD4 tissue-resident memory T cells mediate adaptive immunity induced by previous infection of mice with bordetella pertussis. J Immunol. 2017;199:233–43.
Hosang L, Canals RC, van der Flier FJ, Hollensteiner J, Daniel R, Flugel A, et al. The lung microbiome regulates brain autoimmunity. Nature. 2022;603:138–44.
Odoardi F, Sie C, Streyl K, Ulaganathan VK, Schlager C, Lodygin D, et al. T cells become licensed in the lung to enter the central nervous system. Nature. 2012;488:675–9.
McManus RM, Higgins SC, Mills KH, Lynch MA. Respiratory infection promotes T cell infiltration and amyloid-beta deposition in APP/PS1 mice. Neurobiol Aging. 2014;35:109–21.
Homeyer MA, Falck A, Li LY, Pruss H. From immunobiology to intervention: pathophysiology of autoimmune encephalitis. Semin Immunol. 2025;78:101955.
Machhi J, Yeapuri P, Lu Y, Foster E, Chikhale R, Herskovitz J, et al. CD4+ effector T cells accelerate Alzheimer’s disease in mice. J Neuroinflammation. 2021;18:272.
Jain RW, Yong VW. B cells in central nervous system disease: diversity, locations and pathophysiology. Nat Rev Immunol. 2022;22:513–24.
Beck TC, Gomes AC, Cyster JG, Pereira JP. CXCR4 and a cell-extrinsic mechanism control immature B lymphocyte egress from bone marrow. J Exp Med. 2014;211:2567–81.
Bleul CC, Schultze JL, Springer TA. B lymphocyte chemotaxis regulated in association with microanatomic localization, differentiation state, and B cell receptor engagement. J Exp Med. 1998;187:753–62.
Klein RS, Rubin JB. Immune and nervous system CXCL12 and CXCR4: parallel roles in patterning and plasticity. Trends Immunol. 2004;25:306–14.
Parachikova A, Cotman CW. Reduced CXCL12/CXCR4 results in impaired learning and is downregulated in a mouse model of Alzheimer disease. Neurobiol Dis. 2007;28:143–53.
Song ZH, Song XJ, Yang CL, Cao P, Mao Y, Jin Y, et al. Up-regulation of microglial chemokine CXCL12 in anterior cingulate cortex mediates neuropathic pain in diabetic mice. Acta Pharmacol Sin. 2023;44:1337–49.
Tanabe S, Yamashita T. B-1a lymphocytes promote oligodendrogenesis during brain development. Nat Neurosci. 2018;21:506–16.
Ahn JJ, Islam Y, Clarkson-Paredes C, Karl MT, Miller RH. B cell depletion modulates glial responses and enhances blood vessel integrity in a model of multiple sclerosis. Neurobiol Dis. 2023;187:106290.
Gate D, Saligrama N, Leventhal O, Yang AC, Unger MS, Middeldorp J, et al. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer’s disease. Nature. 2020;577:399–404.
Zhang Y, Fung ITH, Sankar P, Chen X, Robison LS, Ye L, et al. Depletion of NK cells improves cognitive function in the Alzheimer disease mouse model. J Immunol. 2020;205:502–10.
Marsh SE, Abud EM, Lakatos A, Karimzadeh A, Yeung ST, Davtyan H, et al. The adaptive immune system restrains Alzheimer’s disease pathogenesis by modulating microglial function. Proc Natl Acad Sci USA. 2016;113:E1316–25.
Yang H, Park SY, Baek H, Lee C, Chung G, Liu X, et al. Adoptive therapy with amyloid-beta specific regulatory T cells alleviates Alzheimer’s disease. Theranostics. 2022;12:7668–80.
Drieu A, Du S, Storck SE, Rustenhoven J, Papadopoulos Z, Dykstra T, et al. Parenchymal border macrophages regulate the flow dynamics of the cerebrospinal fluid. Nature. 2022;611:585–93.
Martin F, Chan AC. B cell immunobiology in disease: evolving concepts from the clinic. Annu Rev Immunol. 2006;24:467–96.
Lanzavecchia A. Receptor-mediated antigen uptake and its effect on antigen presentation to class II-restricted T lymphocytes. Annu Rev Immunol. 1990;8:773–93.
Avalos AM, Ploegh HL. Early BCR events and antigen capture, processing, and loading on MHC class II on B cells. Front Immunol. 2014;5:92.
Liu B, Luo W, Huang L, Wei C, Huang X, Liu J, et al. Migration inhibition factor secreted by peripheral blood memory B cells binding to CD74-CD44 receptor complex drives macrophage behavior in Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2024;39:15333175241238577.
Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015;42:607–12.
Dowery R, Benhamou D, Benchetrit E, Harel O, Nevelsky A, Zisman-Rozen S, et al. Peripheral B cells repress B-cell regeneration in aging through a TNF-alpha/IGFBP-1/IGF-1 immune-endocrine axis. Blood. 2021;138:1817–29.
Gibson LF, Piktel D, Landreth KS. Insulin-like growth factor-1 potentiates expansion of interleukin-7-dependent pro-B cells. Blood. 1993;82:3005–11.
Alvarez XA, Sampedro C, Cacabelos R, Linares C, Aleixandre M, Garcia-Fantini M, et al. Reduced TNF-alpha and increased IGF-I levels in the serum of Alzheimer’s disease patients treated with the neurotrophic agent cerebrolysin. Int J Neuropsychopharmacol. 2009;12:867–72.
Heneka MT, Morgan D, Jessen F. Passive anti-amyloid beta immunotherapy in Alzheimer’s disease-opportunities and challenges. Lancet. 2024;404:2198–208.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82071199, 81970821, 82204365, and 82304466), grants from the Natural Science Foundation of Jiangsu Province (BK20241869), China Postdoctoral Science Foundation (2023T160070 and 2023M740372), and Postdoctoral research funding from Changzhou Medical Center, Nanjing Medical University (CZKYCMCP202302).
Author information
Authors and Affiliations
Contributions
MX, WXF, and YC conceived and conducted the project. MX, WXF, GH, and YC supervised the project. MX, YLZ, and SXD wrote the paper. YLZ, SXD, MC, SYZ, YL, ZW, and YXJ performed the experiments and data analysis. SXD, SJC, ZW, YMW, and MC contributed to mouse models and cell culture. YLZ, SXD, SJC, JPS, and JYG collected patient tissue samples and information. YLZ, SXD, MC, SYZ, YL, ZW, and YXJ contributed to imaging analysis and interpreted the data.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Yl., Ding, Sx., Cao, M. et al. Lung memory B cells ameliorate Alzheimer’s disease-like pathology in 5×FAD mice through the CXCL12-CXCR4 axis. Acta Pharmacol Sin (2025). https://doi.org/10.1038/s41401-025-01667-8
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41401-025-01667-8