Abstract
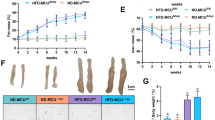
Major urinary protein 3 (Mup3), belonging to the Mup family, is involved in metabolic regulation, but the exact regulatory pathways remain to be elucidated. In this study we investigated the function of Mup3 in regulating hepatic glucose and lipid metabolism. We established four mouse models of metabolic disorders, i.e. db/db and ob/ob obese mice, high-fat diet (HFD)-induced obese (DIO) mice and mice with methionine-choline-deficient (MCD) diet-induced metabolic-associated steatohepatitis (MASH). We found that the expression levels of Mup3 were significantly reduced in the livers of all the four model mice. Moreover, upregulation of Mup3 levels in the liver of HFD-induced obese mice and db/db mice via adeno-associated virus notably decreased blood glucose levels and hepatic triglyceride (TG) content, and improved glucose tolerance and insulin sensitivity. Conversely, Mup3 gene knockout exacerbated HFD-induced hyperglycemia and hepatic lipid accumulation and worsened glucose intolerance and insulin resistance (IR). Restoring the expression of Mup3 reversed these effects in the livers of Mup3-/-. By conducting RNA sequencing (RNA-seq) analysis we revealed that Mup3 primarily modulated gluconeogenesis and the PI3K/AKT signaling cascades. We demonstrated that Mup3 downregulated the genes associated with hepatic gluconeogenesis and lipid synthesis while attenuating hepatic inflammation. These results suggest that Mup3 plays a central role in regulating glucose and lipid balance, highlighting its significance as a prospective treatment target for metabolic disorders, including diabetes and metabolic dysfunction-associated fatty liver disease (MAFLD).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American association for the study of liver diseases. Hepatology. 2018;67:328–57.
Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American gastroenterological association, American association for the study of liver diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–609.
Woo Baidal JA, Lavine JE. The intersection of nonalcoholic fatty liver disease and obesity. Sci Transl Med. 2016;8:323rv1.
Samuel VT, Shulman GI. Nonalcoholic fatty liver disease as a nexus of metabolic and hepatic diseases. Cell Metab. 2018;27:22–41.
Golabi P, Paik JM, AlQahtani S, Younossi Y, Tuncer G, Younossi ZM. Burden of non-alcoholic fatty liver disease in Asia, the middle East and north Africa: data from global burden of disease 2009-2019. J Hepatol. 2021;75:795–809.
Andreasen CR, Andersen A, Vilsbøll T. The future of incretins in the treatment of obesity and non-alcoholic fatty liver disease. Diabetologia. 2023;66:1846–58.
Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836–46.
Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908–22.
Huang X, Liu G, Guo J, Su Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci. 2018;14:1483–96.
Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184:2537–64.
Kasper P, Martin A, Lang S, Kütting F, Goeser T, Demir M, et al. NAFLD and cardiovascular diseases: a clinical review. Clin Res Cardiol J Ger Card Soc. 2021;110:921–37.
Mantovani A, Dalbeni A, Beatrice G, Cappelli D, Gomez-Peralta F. Non-alcoholic fatty liver disease and risk of macro- and microvascular complications in patients with type 2 diabetes. J Clin Med. 2022;11:968.
Marušić M, Paić M, Knobloch M, Liberati Pršo AM. NAFLD, insulin resistance, and diabetes mellitus type 2. Can J Gastroenterol Hepatol. 2021;2021:6613827.
Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279:32345–53.
Greve S, Kuhn GA, Saenz-de-Juano MD, Ghosh A, von Meyenn F, Giller K. The major urinary protein gene cluster knockout mouse as a novel model for translational metabolism research. Sci Rep. 2022;12:13161.
Pallauf K, Günther I, Chin D, Rimbach G. In contrast to dietary restriction, application of resveratrol in mice does not alter mouse major urinary protein expression. Nutrients. 2020;12:815.
Zhou Y, Jiang L, Rui L. Identification of MUP1 as a regulator for glucose and lipid metabolism in mice. J Biol Chem. 2009;284:11152–9.
Gao R, Wang H, Li T, Wang J, Ren Z, Cai N, et al. Secreted MUP1 that reduced under ER stress attenuates ER stress induced insulin resistance through suppressing protein synthesis in hepatocytes. Pharmacol Res. 2023;187:106585.
Zhang H, Li C, Han L, Xiao Y, Bian J, Liu C, et al. MUP1 mediates urolithin A alleviation of chronic alcohol-related liver disease via gut-microbiota-liver axis. Gut Microbes. 2024;16:2367342.
Zhang Y, Wu X, Xu M, Yue T, Ling P, Fang T, et al. Comparative proteomic pnalysis of liver tissues and serum in db/db mice. Int J Mol Sci. 2022;23:9687.
Szoka PR, Gallagher JF, Held WA. In vitro synthesis and characterization of precursors to the mouse major urinary proteins. J Biol Chem. 1980;255:1367–73.
Dhahbi JM, Kim HJ, Mote PL, Beaver RJ, Spindler SR. Temporal linkage between the phenotypic and genomic responses to caloric restriction. Proc Natl Acad Sci USA. 2004;101:5524–9.
Giller K, Huebbe P, Doering F, Pallauf K, Rimbach G. Major urinary protein 5, a scent communication protein, is regulated by dietary restriction and subsequent re-feeding in mice. Proc Biol Sci. 2013;280:20130101.
Chen CC, Lee TY, Kwok CF, Hsu YP, Shih KC, Lin YJ, et al. Major urinary protein 1 interacts with cannabinoid receptor type 1 in fatty acid-induced hepatic insulin resistance in a mouse hepatocyte model. Biochem Biophys Res Commun. 2015;460:1063–8.
Huang JF, Tsai PC, Yeh ML, Huang CF, Huang CI, Hsieh MH, et al. Risk stratification of non-alcoholic fatty liver disease across body mass index in a community basis. J Formos Med Assoc. 2020;119:89–96.
Yao Z, Gong Y, Chen W, Shao S, Song Y, Guo H, et al. Upregulation of WDR6 drives hepatic de novo lipogenesis in insulin resistance in mice. Nat Metab. 2023;5:1706–25.
Meex RCR, Watt MJ. Hepatokines: linking nonalcoholic fatty liver disease and insulin resistance. Nat Rev Endocrinol. 2017;13:509–20.
Lu M, Wan M, Leavens KF, Chu Q, Monks BR, Fernandez S, et al. Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nat Med. 2012;18:388–95.
Dong XC, Copps KD, Guo S, Li Y, Kollipara R, DePinho RA, et al. Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metab. 2008;8:65–76.
Cobbina E, Akhlaghi F. Non-alcoholic fatty liver disease (NAFLD) - pathogenesis, classification, and effect on drug metabolizing enzymes and transporters. Drug Metab Rev. 2017;49:197–211.
Watt MJ, Miotto PM, De Nardo W, Montgomery MK. The liver as an endocrine organ-linking NAFLD and insulin resistance. Endocr Rev. 2019;40:1367–93.
Xia J, Sellers LA, Oxley D, Smith T, Emson P, Keverne EB. Urinary pheromones promote ERK/Akt phosphorylation, regeneration and survival of vomeronasal (V2R) neurons. Eur J Neurosci. 2006;24:3333–42.
Gekle M. Renal tubule albumin transport. Annu Rev Physiol. 2005;67:573–94.
Hu J, Zhu XH, Zhang XJ, Wang PX, Zhang R, Zhang P, et al. Targeting TRAF3 signaling protects against hepatic ischemia/reperfusions injury. J Hepatol. 2016;64:146–59.
Zhang Y, Zhang XJ, Li H. Targeting interferon regulatory factor for cardiometabolic diseases: opportunities and challenges. Curr Drug Targets. 2017;18:1754–78.
Chen Z, Yu Y, Cai J, Li H. Emerging molecular targets for treatment of nonalcoholic fatty liver disease. Trends Endocrinol Metab TEM. 2019;30:903–14.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82370878, 81870402, 82200975 and 82300659), Key Research and Development Program of Anhui Province (2022i01020023), Anhui Science Fund for Distinguished Young Scholars (2208085J45), Research Fund of Anhui Institute of Translational Medicine (2022zhyx-C12), and Natural Science Foundation of Anhui Province (2308085QH247).
Author information
Authors and Affiliations
Contributions
XMZ, JYG, and DW contributed equally to this work. HBZ, CBG, and LZ conceived and designed the experiments. XMZ, JYG, DW, LL, XW, XRX, and HG performed the laboratory experiments. HTH, XYW, and YXX completed the data analysis RNA sequencing. HBZ provided funding. XMZ and JYG drafted the original manuscript. All authors contributed to and have approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhang, Xm., Gao, Jy., Wang, D. et al. Mup3 ameliorates the dysregulation of glucose and lipid metabolism in MAFLD. Acta Pharmacol Sin (2025). https://doi.org/10.1038/s41401-025-01668-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41401-025-01668-7