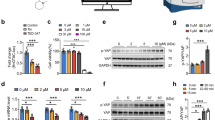
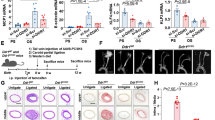
Abstract
Atherosclerosis preferentially develops at arterial bifurcations where the endothelial cells are constantly exposed to disturbed flow, and sustained oscillatory shear stress (OSS) triggers endothelial inflammation. The mechanosensitive transcriptional coactivator YAP plays a critical role in disturbed flow-induced endothelial inflammation. Our recent studies show that disturbed flow upregulates the expression of the mechanosensor 5-HT1B. In this study, we investigated the molecular mechanisms underlying OSS-induced 5-HT1B upregulation in vivo and in vitro. Disturbed flow was induced in mice by partial carotid ligation. In vitro experiments were conducted in human aortic endothelial cells (HAECs) subjected to oscillatory shear stress using an ibidi flow system. We showed that oscillatory shear stress significantly upregulated the expression of 5-HT1B in HAECs via activation of YAP, while knockout of YAP significantly reduced this upregulation. We demonstrated that YAP directly regulated the expression of HTR1B via binding to its promoter region. Inhibition of 5-HT1B using its antagonist SB-216641 impeded YAP nuclear localization and endothelial activation in HAECs. We verified that a 5-HT1B-YAP loop was also activated in atherosclerotic arteries of ApoE−/− mice. Endothelium-specific overexpression of YAP exacerbated atherosclerosis. Moreover, endothelium-specific knockout of 5-HT1B or YAP inhibited disturbed flow-induced endothelial inflammation and plaque formation in ApoE−/− mice. Taken together, the 5-HT1B-YAP positive feedback loop amplifies the pro-atherogenic effect of disturbed flow. We suggest that targeting 5-HT1B-YAP loop holds promise as a novel therapeutic strategy for atherosclerotic diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Mensah GA, Fuster V, Murray CJL, Roth GA. Global burden of cardiovascular diseases and risks, 1990-2022. J Am Coll Cardiol. 2023;82:2350–473.
Luo Y, Liu J, Zeng J, Pan H. Global burden of cardiovascular diseases attributed to low physical activity: an analysis of 204 countries and territories between 1990 and 2019. Am J Prev Cardiol. 2024;17:100633.
Davis MJ, Earley S, Li YS, Chien S. Vascular mechanotransduction. Physiol Rev. 2023;103:1247–421.
Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, et al. Atherosclerosis. Nat Rev Dis Prim. 2019;5:56.
Cheng CK, Wang N, Wang L, Huang Y. Biophysical and biochemical roles of shear stress on endothelium: a revisit and new insights. Circ Res. 2025;136:752–72.
Peng Z, Shu B, Zhang Y, Wang M. Endothelial response to pathophysiological stress. Arterioscler Thromb Vasc Biol. 2019;39:e233–e43.
Immanuel J, Yun S. Vascular inflammatory diseases and endothelial phenotypes. Cells. 2023;12:1640.
Bjorkegren JLM, Lusis AJ. Atherosclerosis: recent developments. Cell. 2022;185:1630–45.
Mitrophanov AY, Groisman EA. Positive feedback in cellular control systems. Bioessays. 2008;30:542–55.
Ratushny AV, Saleem RA, Sitko K, Ramsey SA, Aitchison JD. Asymmetric positive feedback loops reliably control biological responses. Mol Syst Biol. 2012;8:577.
Poston RN. Atherosclerosis: integration of its pathogenesis as a self-perpetuating propagating inflammation: a review. Cardiovasc Endocrinol Metab. 2019;8:51–61.
Tasouli-Drakou V, Ogurek I, Shaikh T, Ringor M, DiCaro MV, Lei K. Atherosclerosis: a comprehensive review of molecular factors and mechanisms. Int J Mol Sci. 2025;26:1364.
Cui X, Wang Y, Li X, Li H, Yin R, Liu Y, et al. A positive feedback loop between CXCL16 and the inflammatory factors IL-17A and TGF-beta promotes large artery atherosclerosis by activating the STAT3/NF-kappaB pathway. Cardiovasc Ther. 2025;2025:2973633.
Feaver RE, Gelfand BD, Wang C, Schwartz MA, Blackman BR. Atheroprone hemodynamics regulate fibronectin deposition to create positive feedback that sustains endothelial inflammation. Circ Res. 2010;106:1703–11.
Xie Q, Li F, Shen K, Luo C, Song G. LOXL1-AS1/miR-515-5p/STAT3 positive feedback loop facilitates cell proliferation and migration in atherosclerosis. J Cardiovasc Pharmacol. 2020;76:151–8.
Kotla S, Zhang A, Imanishi M, Ko KA, Lin SH, Gi YJ, et al. Nucleus-mitochondria positive feedback loop formed by ERK5 S496 phosphorylation-mediated poly (ADP-ribose) polymerase activation provokes persistent pro-inflammatory senescent phenotype and accelerates coronary atherosclerosis after chemo-radiation. Redox Biol. 2021;47:102132.
An T, Guo M, Fan C, Huang S, Liu H, Liu K, et al. sFgl2-Treg positive feedback pathway protects against atherosclerosis. Int J Mol Sci. 2023;24:2338.
Zhu T, Mu BR, Li B, Ran Z, Wang DM, Zhou Y, et al. IGFBP7: a novel biomarker involved in a positive feedback loop with TGF-beta1 in idiopathic pulmonary fibrosis. Cell Signal. 2025;133:111867.
Chen H, Gong Y, Wu F, Wu M, Li S, Chen B, et al. WWP1-SHARP1-C/EBPbeta positive feedback loop modulates development of metabolic dysfunction-associated steatotic liver disease. Metabolism. 2025;169:156271.
Yang TT, Shao YT, Cheng Q, He YT, Qiu Z, Pan DD, et al. YY1/HIF-1alpha/mROS positive-feedback loop exacerbates glomerular mesangial cell proliferation in mouse early diabetic kidney disease. Acta Pharmacol Sin. 2025;46:1974–89.
Ku D, Yang Y, Park Y, Jang D, Lee N, Lee YK, et al. SLIRP amplifies antiviral signaling via positive feedback regulation and contributes to autoimmune diseases. Cell Rep. 2025;44:115588.
Song Z, Gui S, Rao X, Zhang G, Cheng Y, Zeng T. TAZ/NRF2 positive feedback loop contributes to proliferation in bladder cancer through antagonistic ferroptosis. Cell Death Discov. 2025;11:208.
Wang L, Luo JY, Li B, Tian XY, Chen LJ, Huang Y, et al. Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature. 2016;540:579–82.
Wang KC, Yeh YT, Nguyen P, Limqueco E, Lopez J, Thorossian S, et al. Flow-dependent YAP/TAZ activities regulate endothelial phenotypes and atherosclerosis. Proc Natl Acad Sci USA. 2016;113:11525–30.
Jiang M, Ding H, Huang Y, Lau CW, Guo Y, Luo J, et al. Endothelial serotonin receptor 1B acts as a mechanosensor to drive atherosclerosis. Circ Res. 2025;136:887–901.
Ding H, Jiang M, Chan AM, Xia Y, Ma RCW, Yao X, et al. Targeting the tyrosine kinase Src in endothelium attenuates inflammation and atherogenesis induced by disturbed flow. Br J Pharmacol. 2025;182:4861–75.
Jiang MC, Ding HY, Huang YH, Cheng CK, Lau CW, Xia Y, et al. Thioridazine protects against disturbed flow-induced atherosclerosis by inhibiting RhoA/YAP-mediated endothelial inflammation. Acta Pharmacol Sin. 2023;44:1977–88.
Ding H, Jiang M, Lau CW, Luo J, Chan AM, Wang L, et al. Curaxin CBL0137 inhibits endothelial inflammation and atherogenesis via suppression of the Src-YAP signalling axis. Br J Pharmacol. 2023;180:1168–85.
Varadi K, Michelfelder S, Korff T, Hecker M, Trepel M, Katus HA, et al. Novel random peptide libraries displayed on AAV serotype 9 for selection of endothelial cell-directed gene transfer vectors. Gene Ther. 2012;19:800–9.
Zolotukhin S, Byrne BJ, Mason E, Zolotukhin I, Potter M, Chesnut K, et al. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999;6:973–85.
Grieger JC, Choi VW, Samulski RJ. Production and characterization of adeno-associated viral vectors. Nat Protoc. 2006;1:1412–28.
Virani SS, Newby LK, Arnold SV, Bittner V, Brewer LC, Demeter SH, et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA guideline for the management of patients with chronic coronary disease: a report of the American Heart Association/American College of Cardiology joint committee on clinical practice guidelines. Circulation. 2023;148:e9–e119.
Newman CB, Preiss D, Tobert JA, Jacobson TA, Page RL 2nd, Goldstein LB, et al. Statin safety and associated adverse events: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2019;39:e38–e81.
Ward NC, Watts GF, Eckel RH. Statin toxicity. Circ Res. 2019;124:328–50.
Pereira NL, Cresci S, Angiolillo DJ, Batchelor W, Capers QT, Cavallari LH, et al. CYP2C19 genetic testing for oral P2Y12 inhibitor therapy: a scientific statement from the American Heart Association. Circulation. 2024;150:e129–e50.
Jin H, Song J, Shen X, Liang Q, Sun G, Yu Y. Multiple genetic mutations increase the risk of thrombosis associated with clopidogrel after percutaneous coronary intervention. Pharmacogenomics. 2023;24:227–37.
Kong P, Cui ZY, Huang XF, Zhang DD, Guo RJ, Han M. Inflammation and atherosclerosis: signaling pathways and therapeutic intervention. Signal Transduct Target Ther. 2022;7:131.
Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with Canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–31.
Woxholt S, Ueland T, Aukrust P, Anstensrud AK, Broch K, Tollefsen IM, et al. Effect of tocilizumab on endothelial and platelet-derived CXC-chemokines and their association with inflammation and myocardial injury in STEMI patients undergoing primary PCI. Int J Cardiol. 2025;418:132613.
Schmitt C, Abt M, Ciorciaro C, Kling D, Jamois C, Schick E, et al. First-in-Man study with Inclacumab, a human monoclonal antibody against P-selectin. J Cardiovasc Pharmacol. 2015;65:611–9.
Jiang M, Ding H, Huang Y, Wang L. Shear stress and metabolic disorders-two sides of the same plaque. Antioxid Redox Signal. 2022;37:820–41.
Wang Y, Sun Y, Zhao W, Zhang J, Wang X, Hu F, et al. Regulatory mechanisms of the Hippo/YAP axis by G-protein coupled estrogen receptor in gastric signet-ring cell carcinoma. Neoplasia. 2025;67:101199.
Ong YT, Andrade J, Armbruster M, Shi C, Castro M, Costa ASH, et al. A YAP/TAZ-TEAD signalling module links endothelial nutrient acquisition to angiogenic growth. Nat Metab. 2022;4:672–82.
Rauluseviciute I, Riudavets-Puig R, Blanc-Mathieu R, Castro-Mondragon JA, Ferenc K, Kumar V, et al. JASPAR 2024: 20th anniversary of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2024;52:D174–D82.
Abramson J, Adler J, Dunger J, Evans R, Green T, Pritzel A, et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature. 2024;630:493–500.
Alhosaini K, Azhar A, Alonazi A, Al-Zoghaibi F. GPCRs: the most promiscuous druggable receptor of the mankind. Saudi Pharm J. 2021;29:539–51.
Acknowledgements
This research was supported by Hong Kong Research Grants Council grants (T12-101/23-N, SRFS2021-4S04, AoE/M-401/24-R, and 11103222), National Natural Science Foundation of China (91939302), and Hong Kong Health and Medical Research Fund (07181286). The schematic diagrams were prepared using BioRender.
Author information
Authors and Affiliations
Contributions
MCJ, HYD, and YH designed the study. MCJ and HYD conducted experiments and collected data. MCJ, HYD, CKC, DQC, and LW analyzed the data. MCJ, HYD, and YH drafted the manuscript. HXG, JK, RCWM, YX, and XQY provided constructive suggestions in experimental design and helped revise the article. YH, YX, and RCWM provided funds. YH is the leading principal investigator who directed the study. All authors revised and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, Mc., Ding, Hy., Cheng, C.K. et al. Targeting the 5-HT1B-YAP positive feedback loop protects against disturbed flow-induced atherogenesis in mice. Acta Pharmacol Sin (2025). https://doi.org/10.1038/s41401-025-01672-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41401-025-01672-x