Abstract
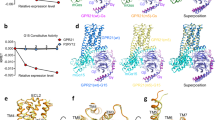
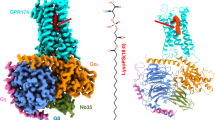
The global obesity epidemic, affecting over 650 million adults, demands innovative therapeutics. GPR75 has emerged as a promising anti-obesity target, with genetic evidence linking loss-of-function variants to protection against obesity and type 2 diabetes. However, structural insights have remained elusive due to GPR75’s inherent expression and stabilization challenges. Here we present the cryo-EM structures of human GPR75 in apo and Gq-coupled states, achieved through advanced stabilization techniques including NanoBiT and molecular glue approaches. Our structures reveal unique architectural features: a completely collapsed extracellular domain eliminates the traditional orthosteric binding pocket, raising critical questions about previously reported small molecule ligands. GPR75 assumes active-like conformation in both apo and G protein complexed structures through unique molecular switches—the canonical DRY motif is replaced by HRL, abolishing the ionic lock, while a distinctive Lys134-Asp210 salt bridge stabilizes the active conformation without ligand binding. This dramatic structural divergence from conventional GPCRs necessitates alternative therapeutic strategies targeting allosteric sites or protein-protein interactions rather than orthosteric pockets. Our findings establish a crucial structural framework for developing next-generation anti-obesity therapeutics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The atomic coordinates of complex, GPR75-Gq complex, and Apo-GPR75BRIL are deposited at Protein Data Bank under access codes 9XQC, and 9XQN, respectively. Cryo-EM density maps of GPR75-Gq complex, and Apo-GPR75BRIL complex are deposited at Electron Microscopy Data Bank under access numbers EMD-67110, and EMD-67119, respectively.
References
Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023;402:203–34.
Powell-Wiley TM, Poirier P, Burke LE, Després JP, Gordon-Larsen P, Lavie CJ, et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;143: e984–e1010.
Abdelaal M, le Roux CW, Docherty NG. Morbidity and mortality associated with obesity. Ann Transl Med. 2017;5:161.
Cho YY, Kim S, Kim P, Jo MJ, Park SE, Choi Y, et al. G-Protein-Coupled Receptor (GPCR) signaling and pharmacology in metabolism: physiology, mechanisms, and therapeutic potential. Biomolecules. 2015;15:291.
Barella LF, Jain S, Pydi SP. G protein-coupled receptors: role in metabolic disorders. Front Endocrinol (Lausanne). 2022;13:984253.
Venniyoor A. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387:1433–4.
Aronne LJ, Horn DB, le Roux CW, Ho W, Falcon BL, Gomez Valderas E, et al. Tirzepatide as compared with semaglutide for the treatment of obesity. N Engl J Med. 2025;393:26–36.
Gorgojo-Martínez JJ, Mezquita-Raya P, Carretero-Gómez J, Castro A, Cebrián-Cuenca A, de Torres-Sánchez A, et al. Clinical recommendations to manage gastrointestinal adverse events in patients treated with Glp-1 receptor agonists: a multidisciplinary expert consensus. J Clin Med. 2022;12:145.
Akbari P, Gilani A, Sosina O, Kosmicki JA, Khrimian L, Fang YY, et al. Sequencing of 640,000 exomes identifies GPR75 variants associated with protection from obesity. Science. 2021;373:eabf8683.
Zhao Y, Chukanova M, Kentistou KA, Fairhurst-Hunter Z, Siegert AM, Jia RY, et al. Protein-truncating variants in BSN are associated with severe adult-onset obesity, type 2 diabetes and fatty liver disease. Nat Genet. 2024;56:579–84.
Hossain S, Gilani A, Pascale J, Villegas E, Diegisser D, Agostinucci K, et al. Gpr75-deficient mice are protected from high-fat diet-induced obesity. Obesity (Silver Spring). 2023;31:1024–37.
Jiang Y, Xun Y, Zhang Z. Central regulation of feeding and body weight by ciliary GPR75. J Clin Invest. 2024;134:e182121.
Leeson-Payne A, Iyinikkel J, Malcolm C, Lam BYH, Sommer N, Dowsett GKC, et al. Loss of GPR75 protects against non-alcoholic fatty liver disease and body fat accumulation. Cell Metab. 2024;36:1076–87.
Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond). 2008;32:1431–7.
Han J, Li J, Yao S, Wei Z, Jiang H, Xu T, et al. GPR75: advances, challenges in deorphanization, and potential as a novel drug target for disease treatment. Int J Mol Sci. 2025;26:4084.
Atanes P, Ashik T, Persaud SJ. Obesity-induced changes in human islet G protein-coupled receptor expression: implications for metabolic regulation. Pharmacol Ther. 2021;228:107928.
Dashti MR, Gorbanzadeh F, Jafari-Gharabaghlou D, Farhoudi Sefidan Jadid M, Zarghami N. G protein-coupled receptor 75 (GPR75) as a novel molecule for targeted therapy of cancer and metabolic syndrome. Asian Pac J Cancer Prev. 2023;24:1817–25.
Lu S, Jang W, Inoue A, Lambert NA. Constitutive G protein coupling profiles of understudied orphan GPCRs. PLoS One. 2021;16:e0247743.
Garcia V, Gilani A, Shkolnik B, Pandey V, Zhang FF, Dakarapu R, et al. 20-HETE signals through G-protein-coupled receptor GPR75 (Gq) to affect vascular function and trigger hypertension. Circ Res. 2017;120:1776–88.
Tunyasuvunakool K, Adler J, Wu Z, Green T, Zielinski M, Žídek A, et al. Highly accurate protein structure prediction for the human proteome. Nature. 2021;596:590–6.
Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–32.
Adams PD, Gopal K, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, et al. Recent developments in the PHENIX software for automated crystallographic structure determination. J Synchrotron Radiat. 2004;11:53–5.
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, et al. UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–12.
He XH, You CZ, Jiang HL, Jiang Y, Xu HE, Cheng X. AlphaFold2 versus experimental structures: evaluation on G protein-coupled receptors. Acta Pharmacol Sin. 2023;44:1–7.
Pándy-Szekeres G, Caroli J, Mamyrbekov A, Kermani AA, Keserű GM, Kooistra AJ, et al. GPCRdb in 2023: state-specific structure models using AlphaFold2 and new ligand resources. Nucleic Acids Res. 2023;51:D395–D402.
Cheng L, Xia F, Li Z, Shen C, Yang Z, Hou H, et al. Structure, function and drug discovery of GPCR signaling. Mol Biomed. 2023;4:46.
Duan J, Xu P, Luan X, Ji Y, He X, Song N, et al. Hormone- and antibody-mediated activation of the thyrotropin receptor. Nature. 2022;609:854–9.
Zhang X, Schlimgen RR, Singh S, Tomani MP, Volkman BF, Zhang C. Molecular basis for chemokine recognition and activation of XCR1. Proc Natl Acad Sci USA. 2024;121:e2405732121.
Duan J, Xu P, Zhang H, Luan X, Yang J, He X, et al. Mechanism of hormone and allosteric agonist mediated activation of follicle stimulating hormone receptor. Nat Commun. 2023;14:519.
Liu H, Deepak RNVK, Shiriaeva A, Gati C, Batyuk A, Hu H, et al. Molecular basis for lipid recognition by the prostaglandin D2 receptor CRTH2. Proc Natl Acad Sci USA. 2021;118:e2102813118.
Xu J, Xu Y, Hou L, He X, Li Y, Zhao J, et al. Molecular basis of lipid and ligand regulation of prostaglandin receptor DP2. Proc Natl Acad Sci USA. 2024;121:e2403304121.
Wu C, Xu Y, He Q, Li D, Duan J, Li C, et al. Ligand-induced activation and G protein coupling of prostaglandin F2α receptor. Nat Commun. 2023;14:2668.
Jiang Y, Zhang Z. Adopting GPR75 in treating obesity: unraveling the knowns and unknowns of this orphan GPCR. Trends Cell Biol. 2025;35:102–4.
Acknowledgements
The cryo-EM data were collected at the Shanghai Advanced Center for Electron Microscopy, Shanghai Institute of Materia Medica, Chinese Academy of Sciences. The National Key R&D Program of China (2022YFA1302900 to WCY; 2022YFC2703105 to HEX); the National Natural Science Foundation of China (32301016 to CRW, 32130022, 82495184, 82121005 to HEX, 82404881 to QNY); National Key R&D Program “Strategic Scientific and Technological Innovation Cooperation” Key Project (2022YFE0203600) released by the Ministry of Science and Technology; CAS Strategic Priority Research Program (XDB37030103 to HEX); Shanghai Municipal Science and Technology Major Project (2019SHZDZX02 to HEX); Shanghai Municipal Science and Technology Major Project (HEX); Supported by the Strategic Priority Research Program of the Chinese Academy of Sciences, Grant No. XDB0830000 to HEX.
We thank Claude 4.5 (Anthropic) for assistance with manuscript editing and language refinement.
Author information
Authors and Affiliations
Contributions
ZNZ, CZY, and QNY contributed equally to this work. CZY expressed and purified the GPR75-Gq complexes and prepared cryo-EM samples. ZNZ expressed and purified the apo GPR75 complexes and prepared cryo-EM samples. QNY and WH collected cryo-EM data. QNY, CRW, and CZY performed cryo-EM data processing and image analysis. QNY and CRW built and refined the atomic models. JJW, JYX, ZYG, KW, WCY, and YWX provided experimental assistance and technical support. ZH, ML, and BS provided scientific advice and contributed to data analysis. HEX and CRW conceived and designed the project, supervised all aspects of the research, analyzed the data, and wrote the manuscript with input from all authors. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
ZH, ML, and BS are employed by BioFront Therapeutics. HEX is one of the Associate Editors of APS and was not involved in the peer review or the decision making of the article. The authors declare no other competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, Zn., You, Cz., Yuan, Qn. et al. Cryo-EM structures of GPR75 reveal an occluded orthosteric pocket challenging conventional drug discovery paradigms for an anti-obesity target. Acta Pharmacol Sin (2026). https://doi.org/10.1038/s41401-025-01720-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41401-025-01720-6