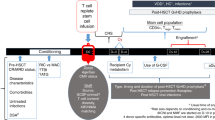
Abstract
In patients with hematological malignancies at high risk for relapse, a mismatched hematopoietic stem cells transplants can be offered with no undue delay between decision-making and transplantation as virtually all patients have a full-haplotype mismatched member who could serve immediately as a donor. Using a T-cell depletion approach, these patients can benefit from a graft-vs-leukemia effect in the absence of both acute and chronic graft-vs-host disease. Over the past decade, efforts have concentrated on developing new conditioning regimens, optimizing the graft processing and improving the posttransplant immunological recovery. The innovative strategy based on the selective depletion of alpha/beta-positive T lymphocytes from G-CSF-mobilized peripheral blood precursor cells has shown very promising results in the setting of the pediatric transplantation. This paper reports the outcome in adult patients with hematological malignancies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Aversa F, Tabilio A, Velardi A, Cunningham I, Terenzi A, Falzetti F, et al. Treatment of high risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N Engl J Med. 1998;339:1186–93.
Gur H, Krauthgamer R, Bachar-Lustig E, Katchman H, Arbel-Goren R, Berrebi A, et al. Immune regulatory activity of CD34+ progenitor cells: evidence for a deletion-based mechanism mediated by TNF-alpha. Blood. 2005;105:2585–93.
Aversa F, Terenzi A, Tabilio A, Falzetti F, Carotti A, Ballanti S, et al. Full-haplotype mismatched hematopoietic stem cell transplantation: a phase II study in patients with acute leukemia at high risk or relapse. J Clin Oncol. 2005;23:3447–54.
Aversa F, Martelli MF, Velardi A. Haploidentical hematopoietic stem cell transplantation with a megadose T-cell-depleted graft: harnessing natural and adaptive immunity. Semin Oncol. 2012;39:643–52.
Handgretinger R. New approaches to graft engineering for haploidentical bone marrow transplantation. Semin Oncol. 2012;39:664–73.
Bertaina A, Merli P, Rutella S, Pagliara D, Bernardo ME, Masetti R, et al. HLA-haploidentical stem cell transplantation after removal of aß+ T and B cells in children with nonmalignant disorders. Blood. 2014;124:822–6.
Airoldi I, Bertaina A, Prigione I, Zorzoli A, Pagliara D, Cocco C, et al. T-cell reconstitution after HLA-haploidentical hematopoietic transplantation depleted of TCR-aß+/CD19+ lymphocytes. Blood. 2015;125:2349–58.
Ruggeri L, Mancusi A, Burchielli E, Aversa F, Martelli MF, Velardi A. Natural killer cell alloreactivity in allogeneic hematopoietic transplantation. Curr Opin Oncol. 2007;19:142–7.
Funding
Publication of this supplement was sponsored by the Gilead Sciences Europe Ltd., Cell Source, Inc., The Chorafas Institute for Scientific Exchange of the Weizmann Institute of Science, Kiadis Pharma, Miltenyi Biotec, Celgene, Centro Servizi Congressuali, Almog Diagnostic.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
FR received lecture fees from Gilead. MC received lecture fees from Novartis, Incyte, and Janssen. NG received consulting fees from Janssen, Celgene, Takeda, Amgen; lecture fees from Janssen, Celgene, Bristol Myers Squibb; and grant support from Janssen Pharmaceuticals, Millennium Pharmaceuticals (Takeda Oncology), and GlaxoSmithKline S.p.A. FA received lecture fees from Gilead, Basilea, Novartis, MSD, Kaidis, and Pfizer. The remaining authors declared no competing interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Prezioso, L., Manfra, I., Bonomini, S. et al. Haploidentical hematopoietic stem cell transplantation in adults using the αβTCR/CD19-based depletion of G-CSF-mobilized peripheral blood progenitor cells. Bone Marrow Transplant 54 (Suppl 2), 698–702 (2019). https://doi.org/10.1038/s41409-019-0608-z
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41409-019-0608-z
This article is cited by
-
TCRαβ/CD19 depleted hematopoietic stem cell transplantation from haploidentical donors: dissecting the GvL/GvHD conundrum
Bone Marrow Transplantation (2020)