Abstract
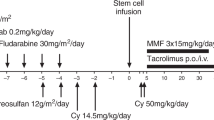
Allogeneic hematopoietic stem cell transplantation (HSCT) is curative for a variety of nonmalignant disorders including osteopetrosis, bone marrow failures, and immune deficiencies. Haploidentical HSCT is a readily available option in the absence of a matched donor, but engraftment failure and other post-transplant complications are a concern. Post-transplant cyclophosphamide (PT-Cy) regimens are gaining popularity and recent reports show promising results. We report our experience with nine pediatric patients with nonmalignant diseases who were transplanted from a haploidentical donor with PT-Cy. From 2015 to 2019, nine children with nonmalignant diseases underwent haploidentical HSCT with PT-Cy, two as a second transplant and seven as primary grafts after upfront serotherapy and busulfan-based myeloablative conditioning. Patient’s diseases included osteopetrosis (n = 5), congenital amegakaryocytic thrombocytopenia (n = 2), hemophagocytic lymphohistiocytosis (n = 1), and Wiskott Aldrich syndrome (n = 1). Two patients failed to engraft following upfront PT-Cy transplants, one was salvaged with a second PT-Cy transplant, and the other with a CD34+ selected graft. None of the patients suffered from graft-versus-host disease. Three patients died from early posttransplant infectious complications and six patients are alive and well. In conclusion, haploidentical HSCT with PT-Cy is a feasible option for pediatric patients with nonmalignant diseases lacking a matched donor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Steward CG. Hematopoietic stem cell transplantation for osteopetrosis. Pediatr Clin NAm. 2010;57:171–80.
Alter BP. Inherited bone marrow failure syndromes: considerations pre- and posttransplant. Hematol Am Soc Hematol Educ Progr. 2017;2017:88–95.
Gennery AR, Albert MH, Slatter MA, Lankester A. Hematopoietic stem cell transplantation for primary immunodeficiencies. Front Pediatr. 2019;7:445.
Gragert L, Eapen M, Williams E, Freeman J, Spellman S, Baitty R, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. 2014;371:339–48.
Mussetti A, Greco R, Peccatori J, Corradini P. Post-transplant cyclophosphamide, a promising anti-graft versus host disease prophylaxis: where do we stand? Expert Rev Hematol. 2017;10:479–92.
Neven B, Diana JS, Castelle M, Magnani A, Rosain J, Touzot F, et al. Haploidentical hematopoietic stem cell transplantation with post-transplant cyclophosphamide for primary immunodeficiencies and inherited disorders in children. Biol Blood Marrow Transplant. 2019;25:1363–73.
Kurzay M, Hauck F, Schmid I, Wiebking V, Eichinger A, Jung E, et al. T-cell replete haploidentical bone marrow transplantation and post-transplant cyclophosphamide for patients with inborn errors. Haematologica. 2019;104:e478–82.
Uppuluri R, Sivasankaran M, Patel S, Swaminathan VV, Ramanan KM, Ravichandran N, et al. Haploidentical stem cell transplantation with post-transplant cyclophosphamide for primary immune deficiency disorders in children: challenges and outcome from a Tertiary Care Center in South India. J Clin Immunol. 2019;39:182–7.
Klein OR, Chen AR, Gamper C, Loeb D, Zambidis E, Llosa N, et al. Alternative-donor hematopoietic stem cell transplantation with post-transplantation cyclophosphamide for nonmalignant disorders. Biol Blood Marrow Transplant. 2016;22:895–901.
Mallhi KK, Srikanthan MA, Baker KK, Frangoul HA, Torgerson TR, Petrovic A et al. HLA-haploidentical hematopoietic cell transplantation for treatment of non-malignant diseases using nonmyeloablative conditioning and post-transplant cyclophosphamide. Biol Blood Marrow Transplant. 2020. https://doi.org/10.1016/j.bbmt.2020.03.018.
Adhikari J, Gyawali B, Sharma P, Bhatt VR. Outcomes of haploidentical transplant compared with matched donor allogeneic stem cell transplant. Future Oncol. 2017;13:935–44.
Guilcher GMT, Shah R, Shenoy S. Principles of alemtuzumab immunoablation in hematopoietic cell transplantation for non-malignant diseases in children: a review. Pediatr Transplant. 2018; 22. https://doi.org/10.1111/petr.13142.
Tolar J, Teitelbaum SL, Orchard PJ. Osteopetrosis. N Engl J Med. 2004;351:2839–49.
Penna S, Capo V, Palagano E, Sobacchi C, Villa A. One disease, many genes: implications for the treatment of osteopetroses. Front Endocrinol. 2019;10:85.
Shadur B, Zaidman I, NaserEddin A, Lokshin E, Hussein F, Oron HC, et al. Successful hematopoietic stem cell transplantation for osteopetrosis using reduced intensity conditioning. Pediatr Blood Cancer. 2018;65:e27010.
Orchard PJ, Fasth AL, Le Rademacher J, He W, Boelens JJ, Horwitz EM, et al. Hematopoietic stem cell transplantation for infantile osteopetrosis. Blood. 2015;126:270–6.
Schulz AS, Classen CF, Mihatsch WA, Sigl-Kraetzig M, Wiesneth M, Debatin K-M, et al. HLA-haploidentical blood progenitor cell transplantation in osteopetrosis. Blood. 2002;99:3458–60.
Pronk CJ, Turkiewicz D, Vult von Steyern K, Ehinger M, Dykes J, Toporski J. Transplantation of haploidentical TcRab-depleted hematopoietic cells allows for optimal timing and sustained correction of the metabolic defect in children with infantile osteopetrosis. J Bone Min Res. 2017;32:82–5.
Fernandes JF, Nichele S, Daudt LE, Tavares RB, Seber A, Kerbauy FR, et al. Transplantation of hematopoietic stem cells for primary immunodeficiencies in brazil: challenges in treating rare diseases in developing countries. J Clin Immunol. 2018;38:917–26.
Bahr TL, Lund T, Sando NM, Orchard PJ, Miller WP. Haploidentical transplantation with post-transplant cyclophosphamide following reduced-intensity conditioning for osteopetrosis: outcomes in three children. Bone Marrow Transplant. 2016;51:1546–8.
Ballmaier M, Germeshausen M. Congenital amegakaryocytic thrombocytopenia: clinical presentation, diagnosis, and treatment. Semin Thromb Hemost. 2011;37:673–81.
Dalle J-H, Peffault de Latour R. Allogeneic hematopoietic stem cell transplantation for inherited bone marrow failure syndromes. Int J Hematol. 2016;103:373–9.
Mahadeo KM, Tewari P, Parikh SH, Driscoll TA, Page K, Martin PL, et al. Durable engraftment and correction of hematological abnormalities in children with congenital amegakaryocytic thrombocytopenia following myeloablative umbilical cord blood transplantation. Pediatr Transplant. 2015;19:753–7.
Canna S, Marsh RA. Pediatric hemophagocytic lymphohistiocytosis (HLH). Blood. 2020. https://doi.org/10.1182/blood.2019000936.
Allen CE, Marsh R, Dawson P, Bollard CM, Shenoy S, Roehrs P, et al. Reduced-intensity conditioning for hematopoietic cell transplant for HLH and primary immune deficiencies. Blood. 2018;132:1438–51.
Kohli S, Rastogi N, Nivargi S, Thakkar D, Katewa S, Yadav SP. Successful haploidentical stem cell transplant with posttransplant cyclophosphamide for hemophagocytic lymphohistiocytosis. J Pediatr Hematol Oncol. 2019;41:e158–60.
Ngwube A, Hanson IC, Orange J, Rider NL, Seeborg F, Shearer W, et al. Outcomes after allogeneic transplant in patients with Wiskott-Aldrich syndrome. Biol Blood Marrow Transplant. 2018;24:537–41.
Yue Y, Shi X, Song Z, Qin J, Li J, Feng S, et al. Posttransplant cyclophosphamide for haploidentical stem cell transplantation in children with Wiskott-Aldrich syndrome. Pediatr Blood Cancer. 2018;65:e27092.
Acknowledgements
We thank our patients and their families for putting their trust in our care and allowing us to publish this paper. We thank our departmental nursing and administrative staff for their devoted work. We also thank Professor Zeev Rotstein, director of Hadassah Medical Center, for his support of our department. Shadur B’s position is supported by the Australian Government Research Training Program Scholarship and Hadassah, Australia. This work was supported by the Deutsche Forschungsgemeinschaft (Discovery and Evaluation of new Combined Immunodeficiency Disease Entities (DECIDE); grant DFG WA 1597/4-2) and the ERA-Net ERARE Consortium EURO-CID.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Even-Or, E., NaserEddin, A., Dinur Schejter, Y. et al. Haploidentical stem cell transplantation with post-transplant cyclophosphamide for osteopetrosis and other nonmalignant diseases. Bone Marrow Transplant 56, 434–441 (2021). https://doi.org/10.1038/s41409-020-01040-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41409-020-01040-9
This article is cited by
-
Metabolic bone disorders and the promise of marine osteoactive compounds
Cellular and Molecular Life Sciences (2024)
-
HLA-haploidentical donor transplants with post-transplant cyclophosphamide in children with primary immune deficiency disorders
Bone Marrow Transplantation (2022)
-
Allogeneic hematopoietic stem cell transplantation for inherited metabolic disorders
International Journal of Hematology (2022)
-
Haploidentical Hematopoietic Cell Transplantation Using Post-transplant Cyclophosphamide for Children with Non-malignant Diseases
Journal of Clinical Immunology (2021)