Abstract
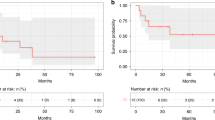
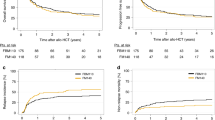
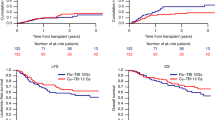
Fludarabine/cyclophosphamide-based conditioning regimens are standard in bone marrow transplantation (BMT) for acquired bone marrow failure in children, however, graft failure may occur. Using the data from a nationwide transplantation registry, we compared the outcomes of children aged <16 years with acquired aplastic anemia and refractory cytopenia of childhood who underwent allogeneic BMT with either fludarabine/melphalan (n = 71) or fludarabine/cyclophosphamide (n = 296) between 2000 and 2016. The fludarabine/melphalan regimen provided excellent outcomes, with 3-year overall survival and failure-free survival rates of 98% and 97%, respectively. The 83% 3-year failure-free survival in the fludarabine/cyclophosphamide group was significantly inferior (P = 0.002), whereas the overall survival did not differ between the two groups. Late graft failure was the most common cause of treatment failure in the fludarabine/cyclophosphamide group, which experienced a significantly higher incidence of late graft failure than the fludarabine/melphalan group (11% vs. 3%; P = 0.035). Multivariate analyses showed that the fludarabine/melphalan regimen was associated with a better failure-free survival (hazard ratio [HR] 0.12; P = 0.005) and lower risk of late graft failure (HR 0.16; P = 0.037). Fludarabine/melphalan-based conditioning regimen can be a promising option for children with acquired bone marrow failure receiving BMT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Camitta BM, Rappeport JM, Parkman R, Nathan DG. Selection of patients for bone marrow transplantation in severe aplastic anemia. Blood. 1975;45:355–63.
Young NS. Acquired aplastic anemia. JAMA. 1999;282:271–8.
Bacigalupo A. How I treat acquired aplastic anemia. Blood. 2017;129:1428–36.
Dufour C, Pillon M, Socie G, Rovo A, Carraro E, Bacigalupo A, et al. Outcome of aplastic anaemia in children. A study by the severe aplastic anaemia and paediatric disease working parties of the European group blood and bone marrow transplant. Br J Haematol. 2015;169:565–73.
Yoshida N, Kobayashi R, Yabe H, Kosaka Y, Yagasaki H, Watanabe K, et al. First-line treatment for severe aplastic anemia in children: bone marrow transplantation from a matched family donor versus immunosuppressive therapy. Haematologica. 2014;99:1784–91.
Dufour C, Veys P, Carraro E, Bhatnagar N, Pillon M, Wynn R, et al. Similar outcome of upfront-unrelated and matched sibling stem cell transplantation in idiopathic paediatric aplastic anaemia. A study on behalf of the UK Paediatric BMT Working Party, Paediatric Diseases Working Party and Severe Aplastic Anaemia Working Party of EBMT. Br J Haematol. 2015;171:585–94.
Kojima S, Inaba J, Yoshimi A, Takahashi Y, Watanabe N, Kudo K, et al. Unrelated donor marrow transplantation in children with severe aplastic anaemia using cyclophosphamide, anti-thymocyte globulin and total body irradiation. Br J Haematol. 2001;114:706–11.
Yagasaki H, Takahashi Y, Hama A, Kudo K, Nishio N, Muramatsu H, et al. Comparison of matched-sibling donor BMT and unrelated donor BMT in children and adolescent with acquired severe aplastic anemia. Bone Marrow Transpl. 2010;45:1508–13.
Storb R, Etzioni R, Anasetti C, Appelbaum FR, Buckner CD, Bensinger W, et al. Cyclophosphamide combined with antithymocyte globulin in preparation for allogeneic marrow transplants in patients with aplastic anemia. Blood. 1994;84:941–9.
Bacigalupo A, Socie G, Lanino E, Prete A, Locatelli F, Locasciulli A, et al. Fludarabine, cyclophosphamide, antithymocyte globulin, with or without low dose total body irradiation, for alternative donor transplants, in acquired severe aplastic anemia: a retrospective study from the EBMT-SAA Working Party. Haematologica. 2010;95:976–82.
Bacigalupo A, Locatelli F, Lanino E, Marsh J, Socie G, Maury S, et al. Fludarabine, cyclophosphamide and anti-thymocyte globulin for alternative donor transplants in acquired severe aplastic anemia: a report from the EBMT-SAA Working Party. Bone Marrow Transpl. 2005;36:947–50.
Marsh JC, Gupta V, Lim Z, Ho AY, Ireland RM, Hayden J, et al. Alemtuzumab with fludarabine and cyclophosphamide reduces chronic graft-versus-host disease after allogeneic stem cell transplantation for acquired aplastic anemia. Blood. 2011;118:2351–7.
Koh LP, Koh MB, Ng HY, Hwang WY, Goh YT, Linn YC, et al. Allogeneic hematopoietic stem cell transplantation for patients with severe aplastic anemia following nonmyeloablative conditioning using 200-cGy total body irradiation and fludarabine. Biol Blood Marrow Transpl. 2006;12:887–90.
Niemeyer CM, Baumann I. Classification of childhood aplastic anemia and myelodysplastic syndrome. Hematology Am Soc Hematol Educ Program. 2011:84–9.
Baumann I, Niemeyer CM, Bennett JM, Shannon K Childhood myelodysplastic syndrome. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, editors. WHO classification of tumors of haematopoietic and lymphoid tissue. 4th ed. IARC Press: Lyon, France; 2008.
Forester CM, Sartain SE, Guo D, Harris MH, Weinberg OK, Fleming MD, et al. Pediatric aplastic anemia and refractory cytopenia: A retrospective analysis assessing outcomes and histomorphologic predictors. Am J Hematol. 2015;90:320–6.
Narita A, Muramatsu H, Sekiya Y, Okuno Y, Sakaguchi H, Nishio N, et al. Paroxysmal nocturnal hemoglobinuria and telomere length predicts response to immunosuppressive therapy in pediatric aplastic anemia. Haematologica. 2015;100:1546–52.
Qin X, Baumann I, Chen J, Shen P, Chen J, Yin M. [Refractory cytopenia of children and acquired aplastic anemia: a clinical and pathological study of 130 cases]. Zhonghua Xue Ye Xue Za Zhi. 2014;35:713–8.
Kojima S. Why is the incidence of aplastic anemia higher in Asia? Expert Rev Hematol. 2017;10:277–9.
Yoshida N, Yagasaki H, Yabe H, Kikuchi A, Kobayashi R, Takahashi Y, et al. Donor-type aplasia after bone marrow transplantation in children with aplastic anemia: a nationwide retrospective study. Blood. 2012;120:959. (abstract)
Yoshida N, Kojima S. Updated guidelines for the treatment of acquired aplastic anemia in children. Curr Oncol Rep. 2018;20:67.
Kato K, Maemura R, Wakamatsu M, Yamamori A, Hamada M, Kataoka S, et al. Allogeneic stem cell transplantation with reduced intensity conditioning for patients with adrenoleukodystrophy. Mol Genet Metab Rep. 2019;18:1–6.
Kudo K, Muramatsu H, Narita A, Yoshida N, Kobayashi R, Yabe H, et al. Unrelated cord blood transplantation in aplastic anemia: is anti-thymocyte globulin indispensable for conditioning? Bone Marrow Transpl. 2017;52:1659–61.
Yamamoto H, Kato D, Uchida N, Ishiwata K, Araoka H, Takagi S, et al. Successful sustained engraftment after reduced-intensity umbilical cord blood transplantation for adult patients with severe aplastic anemia. Blood. 2011;117:3240–2.
Atsuta Y, Suzuki R, Yoshimi A, Gondo H, Tanaka J, Hiraoka A, et al. Unification of hematopoietic stem cell transplantation registries in Japan and establishment of the TRUMP System. Int J Hematol. 2007;86:269–74.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transpl. 1995;15:825–8.
Sullivan KM, Agura E, Anasetti C, Appelbaum F, Badger C, Bearman S, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991;28:250–9.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013;48:452–8.
Bacigalupo A, Marsh JC. Unrelated donor search and unrelated donor transplantation in the adult aplastic anaemia patient aged 18–40 years without an HLA-identical sibling and failing immunosuppression. Bone Marrow Transpl. 2013;48:198–200.
Killick SB, Bown N, Cavenagh J, Dokal I, Foukaneli T, Hill A, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172:187–207.
Anderlini P, Wu J, Gersten I, Ewell M, Tolar J, Antin JH, et al. Cyclophosphamide conditioning in patients with severe aplastic anaemia given unrelated marrow transplantation: a phase 1-2 dose de-escalation study. Lancet Haematol. 2015;2:e367–75.
Tolar J, Deeg HJ, Arai S, Horwitz M, Antin JH, McCarty JM, et al. Fludarabine-based conditioning for marrow transplantation from unrelated donors in severe aplastic anemia: early results of a cyclophosphamide dose deescalation study show life-threatening adverse events at predefined cyclophosphamide dose levels. Biol Blood Marrow Transpl. 2012;18:1007–11.
Larocca A, Piaggio G, Podesta M, Pitto A, Bruno B, Di Grazia C, et al. Boost of CD34+-selected peripheral blood cells without further conditioning in patients with poor graft function following allogeneic stem cell transplantation. Haematologica. 2006;91:935–40.
Kong Y, Chang YJ, Wang YZ, Chen YH, Han W, Wang Y, et al. Association of an impaired bone marrow microenvironment with secondary poor graft function after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transpl. 2013;19:1465–73.
Olsson R, Remberger M, Schaffer M, Berggren DM, Svahn BM, Mattsson J, et al. Graft failure in the modern era of allogeneic hematopoietic SCT. Bone Marrow Transpl. 2013;48:537–43.
Remberger M, Mattsson J, Olsson R, Ringden O. Second allogeneic hematopoietic stem cell transplantation: a treatment for graft failure. Clin Transpl. 2011;25:E68–76.
Stasia A, Ghiso A, Galaverna F, Raiola AM, Gualandi F, Luchetti S, et al. CD34 selected cells for the treatment of poor graft function after allogeneic stem cell transplantation. Biol Blood Marrow Transpl. 2014;20:1440–3.
Mainardi C, Ebinger M, Enkel S, Feuchtinger T, Teltschik HM, Eyrich M, et al. CD34(+) selected stem cell boosts can improve poor graft function after paediatric allogeneic stem cell transplantation. Br J Haematol. 2018;180:90–99.
Shaw A, Passweg JR, De La Fuente J, Bajwa R, Stein J, Al-Zaben A, et al. Relapse of aplastic anemia with majority donor chimerism (donor-type aplasia) occurring late after bone marrow transplantation. Biol Blood Marrow Transplant. 2019. https://doi.org/10.1016/j.bbmt.2019.11.010.
Yang W, Zhang P, Hama A, Ito M, Kojima S, Zhu X. Diagnosis of acquired bone marrow failure syndrome during childhood using the 2008 World Health Organization classification system. Int J Hematol. 2012;96:34–8.
Strahm B, Locatelli F, Bader P, Ehlert K, Kremens B, Zintl F, et al. Reduced intensity conditioning in unrelated donor transplantation for refractory cytopenia in childhood. Bone Marrow Transpl. 2007;40:329–33.
Strahm B, Albert M, Bierings M, Bordon V, Burkhardt B, Catala A, et al. EWOG-MDS study SCT RC RIC 06: Reduced intensity conditioning for children and adolescents with refractory cytopenia of childhood. Bone Marrow Transpl. 2017;52:S103. (abstract)
Howell S, Shalet S. Gonadal damage from chemotherapy and radiotherapy. Endocrinol Metab Clin North Am. 1998;27:927–43.
Ishiguro H, Yasuda Y, Tomita Y, Shinagawa T, Shimizu T, Morimoto T, et al. Gonadal shielding to irradiation is effective in protecting testicular growth and function in long-term survivors of bone marrow transplantation during childhood or adolescence. Bone Marrow Transpl. 2007;39:483–90.
Singhal S, Powles R, Treleaven J, Horton C, Swansbury GJ, Mehta J. Melphalan alone prior to allogeneic bone marrow transplantation from HLA-identical sibling donors for hematologic malignancies: alloengraftment with potential preservation of fertility in women. Bone Marrow Transpl. 1996;18:1049–55.
Kato K, Yoshida N, Matsumoto K, Matsuyama T. Fludarabine, cytarabine, granulocyte colony-stimulating factor and melphalan (FALG with L-PAM) as a reduced toxicity conditioning regimen in children with acute leukemia. Pediatr Blood Cancer. 2014;61:712–6.
Acknowledgements
The authors would like to thank all of the patients and families, and also thank all physicians and members who provided precise data to the Japan Society for Hematopoietic Cell Transplantation. This research was funded by Japanese Red Cross, Nagoya 1st. Hospital Research Grant NFRCH19-0019 (to NY) and was supported in part by the Practical Research Project for Allergic Diseases and Immunology (Research Technology of Medical Transplantation) from the Japan Agency for Medical Research and Development, AMED under grant number 19ek0510023h0002 (to YA).
Pediatric Aplastic Anemia Working Group of the Japan Society for Hematopoietic Cell Transplantation
Nao Yoshida1, Yoshiyuki Takahashi2, Hiromasa Yabe3, Ryoji Kobayashi4, Kenichiro Watanabe5, Kazuko Kudo6, Keisuke Kato19, Hideki Muramatsu2, Atsushi Narita2, Manabu Wakamatsu2, Seiji Kojima2
Author information
Authors and Affiliations
Consortia
Contributions
NY and SK designed research, analyzed and interpreted data, and wrote the manuscript; YT, HY, RK, KW, KKu, and MY interpreted data; TM, KKo, HK, HG, NF, KO, YO, KKa, MI, RS, and YA collected and organized data; all authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Members of the Pediatric Aplastic Anemia Working Group of the Japan Society for Hematopoietic Cell Transplantation are listed below Acknowledgements.
Rights and permissions
About this article
Cite this article
Yoshida, N., Takahashi, Y., Yabe, H. et al. Conditioning regimen for allogeneic bone marrow transplantation in children with acquired bone marrow failure: fludarabine/melphalan vs. fludarabine/cyclophosphamide. Bone Marrow Transplant 55, 1272–1281 (2020). https://doi.org/10.1038/s41409-020-0948-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41409-020-0948-8
This article is cited by
-
Early withdrawal immunosuppression improved mixed chimerism in stem cell transplantation for pediatric aplastic anemia
International Journal of Hematology (2025)
-
Aplastic anemia: history and recent developments in diagnosis and treatment
International Journal of Hematology (2024)
-
Recent advances in the diagnosis and treatment of pediatric acquired aplastic anemia
International Journal of Hematology (2024)