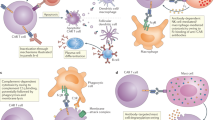
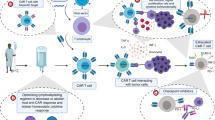
Abstract
CD19-targeted chimeric antigen receptor (CAR) T-cell becomes a breakthrough therapy providing excellent remission rates and durable disease control for patients with relapsed/refractory (R/R) hematologic malignancies. However, CAR T-cells have several potential side effects including cytokine release syndrome, neurotoxicities, cytopenia, and hypogammaglobulinemia. Infection has been increasingly recognized as a complication of CAR T-cell therapy. Several factors predispose CAR T-cell recipients to infection. Fortunately, although studies show a high incidence of infection post-CAR T-cells, most infections are manageable. In contrast to patients who undergo hematopoietic stem cell transplant, less is known about post-CAR T-cell immune reconstitution. Therefore, evidence regarding antimicrobial prophylaxis and vaccination strategies in these patients is more limited. As CAR T-cell therapy becomes the standard treatment for R/R B lymphoid malignancies, we should expect a larger impact of infections in these patients and the need for increased clinical attention. Studies exploring infection and immune reconstitution after CAR T-cell therapy are clinically relevant and will provide us with a better understanding of the dynamics of immune function after CAR T-cell therapy including insights into appropriate strategies for prophylaxis and treatment of infections in these patients. In this review, we describe infections in recipients of CAR T-cells, and discuss risk factors and potential mitigation strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med. 2018;379:64–73.
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–44.
Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56.
Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396:839–52.
Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382:1331–42.
Shah BD, Ghobadi A, Oluwole OO, Logan AC, Boissel N, Cassaday RD, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021;398:491–502.
Jacobson CA, Chavez JC, Sehgal AR, William BM, Munoz J, Salles G, et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022;23:91–103.
Munshi NC, Anderson LD Jr., Shah N, Madduri D, Berdeja J, Lonial S, et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N Engl J Med. 2021;384:705–16.
Berdeja JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398:314–24.
Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127:3321–30.
Shalabi H, Gust J, Taraseviciute A, Wolters PL, Leahy AB, Sandi C, et al. Beyond the storm—subacute toxicities and late effects in children receiving CAR T cells. Nat Rev Clin Oncol. 2021;18:363–78.
Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, et al. Chimeric antigen receptor T-cell therapy—assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15:47–62.
Hill JA, Li D, Hay KA, Green ML, Cherian S, Chen X, et al. Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood. 2018;131:121–30.
Baird JH, Epstein DJ, Tamaresis JS, Ehlinger Z, Spiegel JY, Craig J, et al. Immune reconstitution and infectious complications following axicabtagene ciloleucel therapy for large B-cell lymphoma. Blood Adv. 2021;5:143–55.
Logue JM, Zucchetti E, Bachmeier CA, Krivenko GS, Larson V, Ninh D, et al. Immune reconstitution and associated infections following axicabtagene ciloleucel in relapsed or refractory large B-cell lymphoma. Haematologica. 2021;106:978–86.
Park JH, Romero FA, Taur Y, Sadelain M, Brentjens RJ, Hohl TM, et al. Cytokine release syndrome grade as a predictive marker for infections in patients with relapsed or refractory B-cell acute lymphoblastic leukemia treated with chimeric antigen receptor T cells. Clin Infect Dis. 2018;67:533–40.
Wudhikarn K, Palomba ML, Pennisi M, Garcia-Recio M, Flynn JR, Devlin SM, et al. Infection during the first year in patients treated with CD19 CAR T cells for diffuse large B cell lymphoma. Blood Cancer J. 2020;10:79.
Vora SB, Waghmare A, Englund JA, Qu P, Gardner RA, Hill JA. Infectious complications following CD19 chimeric antigen receptor T-cell therapy for children, adolescents, and young adults. Open Forum Infect Dis. 2020;7:ofaa121.
Mikkilineni L, Yates B, Steinberg SM, Shahani SA, Molina JC, Palmore T, et al. Infectious complications of CAR T-cell therapy across novel antigen targets in the first 30 days. Blood Adv. 2021;5:5312–22.
Hill JA, Seo SK. How I prevent infections in patients receiving CD19-targeted chimeric antigen receptor T cells for B-cell malignancies. Blood. 2020;136:925–35.
Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20:31–42.
Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–48.
Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386:640–54.
Kamdar M, Solomon SR, Arnason JE, Johnston PB, Glass B, Bachanova V, et al. Lisocabtagene Maraleucel (liso-cel), a CD19-Directed Chimeric Antigen Receptor (CAR) T Cell Therapy, Versus Standard of Care (SOC) with Salvage Chemotherapy (CT) Followed By Autologous Stem Cell Transplantation (ASCT) As Second-Line (2L) Treatment in Patients (Pts) with Relapsed or Refractory (R/R) Large B-Cell Lymphoma (LBCL): Results from the Randomized Phase 3 Transform Study. Blood. 2021;138:91.
Neelapu SS, Dickinson M, Munoz J, Ulrickson ML, Thieblemont C, Oluwole OO, et al. Axicabtagene ciloleucel as first-line therapy in high-risk large B-cell lymphoma: the phase 2 ZUMA-12 trial. Nat Med. 2022;28:735–42.
Fowler NH, Dickinson M, Dreyling M, Martinez-Lopez J, Kolstad A, Butler J, et al. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 ELARA trial. Nat Med. 2022;28:325–32.
Bishop MR, Dickinson M, Purtill D, Barba P, Santoro A, Hamad N, et al. Second-line tisagenlecleucel or standard care in aggressive B-cell lymphoma. N Engl J Med. 2022;386:629–39.
Cordeiro A, Bezerra ED, Hirayama AV, Hill JA, Wu QV, Voutsinas J, et al. Late events after treatment with CD19-targeted chimeric antigen receptor modified T cells. Biol Blood Marrow Transpl. 2020;26:26–33.
Wittmann Dayagi T, Sherman G, Bielorai B, Adam E, Besser MJ, Shimoni A, et al. Characteristics and risk factors of infections following CD28-based CD19 CAR-T cells. Leuk Lymphoma. 2021;62:1692–701.
Strati P, Varma A, Adkins S, Nastoupil LJ, Westin J, Hagemeister FB, et al. Hematopoietic recovery and immune reconstitution after axicabtagene ciloleucel in patients with large B-cell lymphoma. Haematologica. 2021;106:2667–72.
Korell F, Schubert ML, Sauer T, Schmitt A, Derigs P, Weber TF, et al. Infection complications after lymphodepletion and dosing of chimeric antigen receptor T (CAR-T) cell therapy in patients with relapsed/refractory acute lymphoblastic leukemia or B cell non-Hodgkin lymphoma. Cancers. 2021;13:1684.
Kambhampati S, Sheng Y, Huang CY, Bylsma S, Lo M, Kennedy V, et al. Infectious complications in patients with relapsed refractory multiple myeloma after BCMA CAR T-cell therapy. Blood Adv. 2022;6:2045–54.
Josyula S, Pont MJ, Dasgupta S, Song X, Thomas S, Pepper G, et al. Pathogen-specific humoral immunity and infections in B Cell maturation antigen-directed chimeric antigen receptor T cell therapy recipients with multiple myeloma. Transplant Cell Ther. 2022;28:304.e1–304.e9.
Wang D, Mao X, Que Y, Xu M, Cheng Y, Huang L, et al. Viral infection/reactivation during long-term follow-up in multiple myeloma patients with anti-BCMA CAR therapy. Blood. Cancer J. 2021;11:168.
Heldman MR, Ma J, Gauthier J, O’Hara RA, Cowan AJ, Yoke LM, et al. CMV and HSV pneumonia after immunosuppressive agents for treatment of cytokine release syndrome due to chimeric antigen receptor-modified T (CAR-T)-cell immunotherapy. J Immunother. 2021;44:351–4.
Hensley MK, Bain WG, Jacobs J, Nambulli S, Parikh U, Cillo A, et al. Intractable Coronavirus Disease 2019 (COVID-19) and prolonged severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) replication in a chimeric antigen receptor-modified T-cell therapy recipient: a case study. Clin Infect Dis. 2021;73:e815–21.
Abid MB, Mughal M, Abid MA. Coronavirus disease 2019 (COVID-19) and immune-engaging cancer treatment. JAMA Oncol. 2020;6:1529–30.
Maurer K, Saucier A, Kim HT, Acharya U, Mo CC, Porter J, et al. COVID-19 and hematopoietic stem cell transplantation and immune effector cell therapy: a US cancer center experience. Blood Adv. 2021;5:861–71.
Aydillo T, Gonzalez-Reiche AS, Aslam S, van de Guchte A, Khan Z, Obla A, et al. Shedding of viable SARS-CoV-2 after immunosuppressive therapy for cancer. N Engl J Med. 2020;383:2586–8.
Busca A, Salmanton-Garcia J, Corradini P, Marchesi F, Cabirta A, Di Blasi R, et al. COVID-19 and CAR-T cells: current challenges and future directions-a report from the EPICOVIDEHA survey by EHA-IDWP. Blood Adv. 2022;6:2427–33.
Spanjaart AM, Ljungman P, de La Camara R, Tridello G, Ortiz-Maldonado V, Urbano-Ispizua A, et al. Poor outcome of patients with COVID-19 after CAR T-cell therapy for B-cell malignancies: results of a multicenter study on behalf of the European Society for Blood and Marrow Transplantation (EBMT) Infectious Diseases Working Party and the European Hematology Association (EHA) Lymphoma Group. Leukemia. 2021;35:3585–8.
Rejeski K, Kunz WG, Rudelius M, Bucklein V, Blumenberg V, Schmidt C, et al. Severe Candida glabrata pancolitis and fatal Aspergillus fumigatus pulmonary infection in the setting of bone marrow aplasia after CD19-directed CAR T-cell therapy—a case report. BMC Infect Dis. 2021;21:121.
Haidar G, Dorritie K, Farah R, Bogdanovich T, Nguyen MH, Samanta P. Invasive mold infections after chimeric antigen receptor-modified T-cell therapy: a case series, review of the literature, and implications for prophylaxis. Clin Infect Dis. 2020;71:672–6.
Maron GM, Hijano DR, Epperly R, Su Y, Tang L, Hayden RT, et al. Infectious complications in pediatric, adolescent and young adult patients undergoing CD19-CAR T cell therapy. Front Oncol. 2022;12:845540.
Shahid S, Ramaswamy K, Flynn J, Mauguen A, Perica K, Park JH, et al. Impact of bridging chemotherapy on clinical outcomes of CD19-specific CAR T cell therapy in children/young adults with relapsed/refractory B cell acute lymphoblastic leukemia. Transpl Cell Ther. 2022;28:72.e1–8.
Perica K, Flynn J, Curran KJ, Rivere I, Wang X, Senechal B, et al. Impact of bridging chemotherapy on clinical outcome of CD19 CAR T therapy in adult acute lymphoblastic leukemia. Leukemia. 2021;35:3268–71.
Jain T, Knezevic A, Pennisi M, Chen Y, Ruiz JD, Purdon TJ, et al. Hematopoietic recovery in patients receiving chimeric antigen receptor T-cell therapy for hematologic malignancies. Blood Adv. 2020;4:3776–87.
Rejeski K, Perez A, Sesques P, Hoster E, Berger C, Jentzsch L, et al. CAR-HEMATOTOX: a model for CAR T-cell-related hematologic toxicity in relapsed/refractory large B-cell lymphoma. Blood. 2021;138:2499–513.
Faramand RG, Davila ML. CAR T-cell hematotoxicity: is inflammation the key? Blood. 2021;138:2447–8.
Schiff MH, Kremer JM, Jahreis A, Vernon E, Isaacs JD, van Vollenhoven RF. Integrated safety in tocilizumab clinical trials. Arthritis Res Ther. 2011;13:R141.
Frigault MJ, Nikiforow S, Mansour MK, Hu ZH, Horowitz MM, Riches ML, et al. Tocilizumab not associated with increased infection risk after CAR T-cell therapy: implications for COVID-19? Blood. 2020;136:137–9.
Neill L, Mackenzie SC, Marzolini MAV, Townsend W, Ardeshna KM, Cwynarski K, et al. Steroid use, advanced stage disease and ≥3 lines of prior chemotherapy are associated with a higher risk of infection following CD19 CAR T-cell therapy for B-NHL: real world data from a large UK center. Blood 2020;136:20–1. %@ 0006-4971
Strati P, Ahmed S, Furqan F, Fayad LE, Lee HJ, Iyer SP, et al. Prognostic impact of corticosteroids on efficacy of chimeric antigen receptor T-cell therapy in large B-cell lymphoma. Blood 2021;137:3272–6.
Garner W, Samanta P, Haidar G. Invasive fungal infections after Anti-CD19 chimeric antigen receptor-modified T-cell therapy: state of the evidence and future directions. J Fungi. 2021;7:156.
Davila ML, Kloss CC, Gunset G, Sadelain M. CD19 CAR-targeted T cells induce long-term remission and B Cell Aplasia in an immunocompetent mouse model of B cell acute lymphoblastic leukemia. PLoS One. 2013;8:e61338.
Doan A, Pulsipher MA. Hypogammaglobulinemia due to CAR T-cell therapy. Pediatr Blood Cancer. 2018;65. https://doi.org/10.1002/pbc.26914.
Walti CS, Krantz EM, Maalouf J, Boonyaratanakornkit J, Keane-Candib J, Joncas-Schronce L, et al. Antibodies against vaccine-preventable infections after CAR-T cell therapy for B cell malignancies. JCI Insight. 2021;6:e146743.
Walti CS, Loes AN, Shuey K, Krantz EM, Boonyaratanakornkit J, Keane-Candib J, et al. Humoral immunogenicity of the seasonal influenza vaccine before and after CAR-T-cell therapy. J Immunother Cancer. 2021;9:e003428.
Wang L, Hong R, Zhou L, Ni F, Zhang M, Zhao H, et al. New-onset severe cytopenia after CAR-T cell therapy: analysis of 76 patients with relapsed or refractory acute lymphoblastic leukemia. Front Oncol. 2021;11:702644.
Fried S, Avigdor A, Bielorai B, Meir A, Besser MJ, Schachter J, et al. Early and late hematologic toxicity following CD19 CAR-T cells. Bone Marrow Transpl. 2019;54:1643–50.
Wudhikarn K, Pennisi M, Garcia-Recio M, Flynn JR, Afuye A, Silverberg ML, et al. DLBCL patients treated with CD19 CAR T cells experience a high burden of organ toxicities but low nonrelapse mortality. Blood Adv. 2020;4:3024–33.
Schaefer A, Saygin C, Maakaron J, Hoelscher T, Purdin Z, Robinson J, et al. Cytopenias after chimeric antigen receptor T-Cells (CAR-T) infusion; patterns and outcomes. Biol Blood Marrow Transplant. 2019;25:S171.
Sharma N, Reagan PM, Liesveld JL. Cytopenia after CAR-T cell therapy—a brief review of a complex problem. Cancers. 2022;14:1501.
Taneja A, Jain T. CAR-T-OPENIA: chimeric antigen receptor T-cell therapy-associated cytopenias. eJHaem. 2022;3:32–8.
Sham M, Andrzej JJ, Myo H, Luciano JC, Kelvin L, Siddhartha G, et al. Orvacabtagene autoleucel (orva-cel), a B-cell maturation antigen (BCMA)-directed CAR T cell therapy for patients (pts) with relapsed/refractory multiple myeloma (RRMM): update of the phase 1/2 EVOLVE study (NCT03430011). J Clin Oncol. 2020;38:8504.
Nagle SJ, Murphree C, Raess PW, Schachter L, Chen A, Hayes-Lattin B, et al. Prolonged hematologic toxicity following treatment with chimeric antigen receptor T cells in patients with hematologic malignancies. Am J Hematol. 2021;96:455–61.
Juluri KR, Hirayama AV, Mullane E, Cleary N, Wu Q, Maloney DG, et al. Factors associated with impaired hematopoietic recovery after CD19-targeted CAR-T cell therapy. Biol Blood Marrow Transplant. 2020;26:S313.
Melenhorst JJ, Chen GM, Wang M, Porter DL, Chen C, Collins MA, et al. Decade-long leukaemia remissions with persistence of CD4(+) CAR T cells. Nature. 2022;602:503–9.
Gardner RA, Finney O, Annesley C, Brakke H, Summers C, Leger K, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129:3322–31.
Shah BD, Bishop MR, Oluwole OO, Logan AC, Baer MR, Donnellan WB, et al. KTE-X19 anti-CD19 CAR T-cell therapy in adult relapsed/refractory acute lymphoblastic leukemia: ZUMA-3 phase 1 results. Blood. 2021;138:11–22.
Uy NF, Pequignot E, Frey NV, Davis M, Hexner EO, Schuster SJ, et al. Hypogammaglobulinemia and infection risk in chronic lymphocytic leukemia (CLL) patients treated with CD19-directed chimeric antigen receptor T (CAR-T) Cells. Blood. 2020;136:30–2.
Hill JA, Krantz EM, Hay KA, Dasgupta S, Stevens-Ayers T, Bender Ignacio RA, et al. Durable preservation of antiviral antibodies after CD19-directed chimeric antigen receptor T-cell immunotherapy. Blood Adv. 2019;3:3590–601.
Bhoj VG, Arhontoulis D, Wertheim G, Capobianchi J, Callahan CA, Ellebrecht CT, et al. Persistence of long-lived plasma cells and humoral immunity in individuals responding to CD19-directed CAR T-cell therapy. Blood. 2016;128:360–70.
Kampouri E, Walti CS, Gauthier J, Hill JA. Managing hypogammaglobulinemia in patients treated with CAR-T-cell therapy: key points for clinicians. Expert Rev Hematol. 2022;15:305–20.
Los-Arcos I, Iacoboni G, Aguilar-Guisado M, Alsina-Manrique L, Diaz de Heredia C, Fortuny-Guasch C, et al. Recommendations for screening, monitoring, prevention, and prophylaxis of infections in adult and pediatric patients receiving CAR T-cell therapy: a position paper. Infection. 2021;49:215–31.
Yakoub-Agha I, Chabannon C, Bader P, Basak GW, Bonig H, Ciceri F, et al. Management of adults and children undergoing chimeric antigen receptor T-cell therapy: best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE). Haematologica. 2020;105:297–316.
Norelli M, Camisa B, Barbiera G, Falcone L, Purevdorj A, Genua M, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24:739–48.
Giavridis T, van der Stegen SJC, Eyquem J, Hamieh M, Piersigilli A, Sadelain MCAR. T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med. 2018;24:731–8.
Galli E, Allain V, Di Blasi R, Bernard S, Vercellino L, Morin F, et al. G-CSF does not worsen toxicities and efficacy of CAR-T cells in refractory/relapsed B-cell lymphoma. Bone Marrow Transpl. 2020;55:2347–9.
Barreto JN, Bansal R, Hathcock MA, Doleski CJ, Hayne JR, Truong TA, et al. The impact of granulocyte colony stimulating factor on patients receiving chimeric antigen receptor T-cell therapy. Am J Hematol. 2021;96:E399–402.
Gaut D, Tang K, Sim MS, Duong T, Young P, Sasine J. Filgrastim associations with CAR T-cell therapy. Int J Cancer. 2021;148:1192–6.
Lievin R, Di Blasi R, Morin F, Galli E, Allain V, De Jorna R, et al. Effect of early granulocyte-colony-stimulating factor administration in the prevention of febrile neutropenia and impact on toxicity and efficacy of anti-CD19 CAR-T in patients with relapsed/refractory B-cell lymphoma. Bone Marrow Transpl. 2022;57:431–9.
Kansagra AJ, Frey NV, Bar M, Laetsch TW, Carpenter PA, Savani BN, et al. Clinical utilization of chimeric antigen receptor T cells in B cell acute lymphoblastic leukemia: an expert opinion from the European Society for Blood and Marrow Transplantation and the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transpl. 2019;25:e76–85.
Schubert ML, Schmitt M, Wang L, Ramos CA, Jordan K, Muller-Tidow C, et al. Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann Oncol. 2021;32:34–48.
Hayden PJ, Roddie C, Bader P, Basak GW, Bonig H, Bonini C, et al. Management of adults and children receiving CAR T-cell therapy: 2021 best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE) and the European Haematology Association (EHA). Ann Oncol. 2022;33:259–75.
Yang C, Xie M, Zhang K, Liu H, Liang A, Young KH, et al. Risk of HBV reactivation post CD19-CAR-T cell therapy in DLBCL patients with concomitant chronic HBV infection. Leukemia. 2020;34:3055–9.
Wei J, Zhu X, Mao X, Huang L, Meng F, Zhou J. Severe early hepatitis B reactivation in a patient receiving anti-CD19 and anti-CD22 CAR T cells for the treatment of diffuse large B-cell lymphoma. J Immunother Cancer. 2019;7:315.
Strati P, Nastoupil LJ, Fayad LE, Samaniego F, Adkins S, Neelapu SS. Safety of CAR T-cell therapy in patients with B-cell lymphoma and chronic hepatitis B or C virus infection. Blood. 2019;133:2800–2.
Cao W, Wei J, Wang N, Xu H, Xiao M, Huang L, et al. Entecavir prophylaxis for hepatitis B virus reactivation in patients with CAR T-cell therapy. Blood. 2020;136:516–9.
Wang Y, Liu Y, Tan X, Pan B, Ge J, Qi K, et al. Safety and efficacy of chimeric antigen receptor (CAR)-T-cell therapy in persons with advanced B-cell cancers and hepatitis B virus-infection. Leukemia. 2020;34:2704–7.
Han L, Zhou J, Zhou K, Zhu X, Zhao L, Fang B, et al. Safety and efficacy of CAR-T cell targeting BCMA in patients with multiple myeloma coinfected with chronic hepatitis B virus. J Immunother Cancer. 2020;8:e000927.
Langerbeins P, Hallek M. COVID-19 in patients with hematologic malignancy. Blood. 2022:blood.2021012251. Online ahead of print.
Levin MJ, Ustianowski A, De Wit S, Launay O, Avila M, Templeton A, et al. Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for prevention of Covid-19. N Engl J Med. 2022;386:2188–200.
Stuver R, Shah GL, Korde NS, Roeker LE, Mato AR, Batlevi CL, et al. Activity of AZD7442 (tixagevimab-cilgavimab) against Omicron SARS-CoV-2 in patients with hematologic malignancies. Cancer Cell. 2022;40:590–1.
Meir J, Abid MA, Abid MB. State of the CAR-T: risk of infections with chimeric antigen receptor T-cell therapy and determinants of SARS-CoV-2 vaccine responses. Transpl Cell Ther. 2021;27:973–87.
Ohmoto A, Fuji S, Shultes KC, Savani BN, Einsele H. Controversies about immunoglobulin replacement therapy in HSCT recipients with hypogammaglobulinemia. Bone Marrow Transplant. 2022;57:874–80.
Raanani P, Gafter-Gvili A, Paul M, Ben-Bassat I, Leibovici L, Shpilberg O. Immunoglobulin prophylaxis in hematopoietic stem cell transplantation: systematic review and meta-analysis. J Clin Oncol. 2009;27:770–81.
Compagno N, Malipiero G, Cinetto F, Agostini C. Immunoglobulin replacement therapy in secondary hypogammaglobulinemia. Front Immunol. 2014;5:626.
Ueda M, Berger M, Gale RP, Lazarus HM. Immunoglobulin therapy in hematologic neoplasms and after hematopoietic cell transplantation. Blood Rev. 2018;32:106–15.
Ahn H, Tay J, Shea B, Hutton B, Shorr R, Knoll GA, et al. Effectiveness of immunoglobulin prophylaxis in reducing clinical complications of hematopoietic stem cell transplantation: a systematic review and meta-analysis. Transfusion 2018;58:2437–52.
Hill JA, Giralt S, Torgerson TR, Lazarus HM. CAR-T - and a side order of IgG, to go?—Immunoglobulin replacement in patients receiving CAR-T cell therapy. Blood Rev. 2019;38:100596.
Wat J, Barmettler S. Hypogammaglobulinemia after chimeric antigen receptor (CAR) T-cell therapy: characteristics, management, and future directions. J Allergy Clin Immunol Pract. 2022;10:460–6.
Carpenter PA, Englund JA. How I vaccinate blood and marrow transplant recipients. Blood. 2016;127:2824–32.
Mikulska M, Cesaro S, de Lavallade H, Di Blasi R, Einarsdottir S, Gallo G, et al. Vaccination of patients with haematological malignancies who did not have transplantations: guidelines from the 2017 European Conference on Infections in Leukaemia (ECIL 7). (1474-4457 (Electronic)).
Abid MB. Overlap of immunotherapy-related pneumonitis and COVID-19 pneumonia: diagnostic and vaccine considerations. J Immunother Cancer. 2021;9:e002307.
American Society of Hematology. COVID-19 resources: ASH-ASTCT COVID-19 vaccination for HCT and CAR T cell recipients [Accessed 25 April 2022]. https://www.hematology.org/covid-19/ash-astct-covid-19-vaccination-for-hct-and-car-t-cell-recipients.
Abid MA, Abid MB. SARS-CoV-2 vaccine response in CAR T-cell therapy recipients: a systematic review and preliminary observations. Hematol Oncol. 2022;40:287–91.
Dhakal B, Abedin S, Fenske T, Chhabra S, Ledeboer N, Hari P, et al. Response to SARS-CoV-2 vaccination in patients after hematopoietic cell transplantation and CAR T-cell therapy. Blood. 2021;138:1278–81.
Tamari R, Politikos I, Knorr DA, Vardhana SA, Young JC, Marcello LT, et al. Predictors of humoral response to SARS-CoV-2 vaccination after hematopoietic cell transplantation and CAR T-cell therapy. Blood Cancer Discov. 2021;2:577–85.
Jarisch A, Wiercinska E, Huenecke S, Bremm M, Cappel C, Hauler J, et al. Immune responses to SARS-CoV-2 vaccination in young patients with anti-CD19 CAR-T-induced B-cell aplasia. Transplant Cell Ther. 2022;28:366.e1–366.e7.
Parvathaneni K, Torres-Rodriguez K, Meng W, Hwang WT, Frey N, Naji A, et al. SARS-CoV-2 spike-specific T-cell responses in patients with B-cell depletion who received chimeric antigen receptor T-cell treatments. JAMA Oncol. 2022;8:164–7.
Acknowledgements
This research was supported in part by NIH/NCI Cancer Center Support Grant P30 CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
KW generated the concept of the study, conducted the literature search, reviewed the literature, and draft the initial manuscript. MAP contributed to writing the manuscript and provided feedback on the manuscript. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
M-AP reports honoraria from Abbvie, Allovir, Astellas, Bristol-Myers Squibb, Celgene, Equilium, Exevir, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Novartis, Nektar Therapeutics, Omeros, OrcaBio, Takeda, and VectivBio AG, Vor Biopharma. He serves on DSMBs for Cidara Therapeutics, Medigene, Sellas Life Sciences, and Servier, and the scientific advisory board of NexImmune. He has ownership interests in NexImmune and Omeros. He has received institutional research support for clinical trials from Incyte, Kite/Gilead, Miltenyi Biotec, Nektar Therapeutics, and Novartis. KW declares no relevant conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wudhikarn, K., Perales, MA. Infectious complications, immune reconstitution, and infection prophylaxis after CD19 chimeric antigen receptor T-cell therapy. Bone Marrow Transplant 57, 1477–1488 (2022). https://doi.org/10.1038/s41409-022-01756-w
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41409-022-01756-w
This article is cited by
-
Emerging strategies to reduce the side effects of CAR-T cell therapy: focusing on gene editing and nanotechnology
Clinical and Translational Oncology (2026)
-
Pharmacovigilance in Cell and Gene Therapy: Evolving Challenges in Risk Management and Long-Term Follow-Up
Drug Safety (2026)
-
From lab to lifesaver: the rise of CAR T-cell therapy in oncology
Journal of the Egyptian National Cancer Institute (2025)
-
Nebenwirkungsmanagement bei CAR-T-Zellen
Die Innere Medizin (2025)
-
Safety and efficacy of CAR-T cell therapy in patients with autoimmune diseases: a systematic review
Rheumatology International (2025)