Abstract
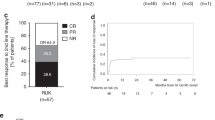
Involvement of lower gastrointestinal tract (LGI) occurs in 60% of patients with graft-versus-host-disease (GVHD). Complement components C3 and C5 are involved in GVHD pathogenesis. In this phase 2a study, we evaluated the safety and efficacy of ALXN1007, a monoclonal antibody against C5a, in patients with newly diagnosed LGI acute GVHD receiving concomitant corticosteroid. Twenty-five patients were enrolled; one was excluded from the efficacy analysis based upon negative biopsy. Most patients (16/25, 64%) had acute leukemia; 52% (13/25) had an HLA-matched unrelated donor; and 68% (17/25) received myeloablative conditioning. Half the patients (12/24) had a high biomarker profile, Ann Arbor score 3; 42% (10/24) had high-risk GVHD per Minnesota classification. Day-28 overall response was 58% (13/24 complete response, 1/24 partial response), and 63% by Day-56 (all complete responses). Day-28 overall response was 50% (5/10) in Minnesota high-risk and 42% (5/12) in high-risk Ann Arbor patients, increasing to 58% (7/12) by Day-56. Non-relapse mortality at 6-months was 24% (95% CI 11–53). The most common treatment-related adverse event was infection (6/25, 24%). Neither baseline complement levels (except for C5), activity, nor inhibition of C5a with ALXN1007 correlated with GVHD severity or responses. Further studies are needed to evaluate the role of complement inhibition in GVHD treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Alexion will consider requests for disclosure of clinical study participant-level data provided that participant privacy is assured through methods like data de-identification, pseudonymization, or anonymization (as required by applicable law), and if such disclosure was included in the relevant study informed consent form or similar documentation. Qualified academic investigators may request participant-level clinical data and supporting documents (statistical analysis plan and protocol) pertaining to Alexion-sponsored studies. Further details regarding data availability and instructions for requesting information are available in the Alexion Clinical Trials Disclosure and Transparency Policy at https://alexionclinicaltrials.com/Disclosure-and-Transparency-Policy. Link to Data Request Form (https://alexion.com/contact-alexion/medical-information).
References
Martin PJ, McDonald GB, Sanders JE, Anasetti C, Appelbaum FR, Deeg HJ, et al. Increasingly frequent diagnosis of acute gastrointestinal graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transpl. 2004;10:320–7.
Nash RA, Antin JH, Karanes C, Fay JW, Avalos BR, Yeager AM, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96:2062–8.
Ratanatharathorn V, Nash RA, Przepiorka D, Devine SM, Klein JL, Weisdorf D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92:2303–14.
McDonald GB. How I treat acute graft-versus-host disease of the gastrointestinal tract and the liver. Blood. 2016;127:1544–50.
Antin JH, Chen AR, Couriel DR, Ho VT, Nash RA, Weisdorf D. Novel approaches to the therapy of steroid-resistant acute graft-versus-host disease. Biol Blood Marrow Transpl. 2004;10:655–68.
Castilla-Llorente C, Martin PJ, McDonald GB, Storer BE, Appelbaum FR, Deeg HJ, et al. Prognostic factors and outcomes of severe gastrointestinal GVHD after allogeneic hematopoietic cell transplantation. Bone Marrow Transpl. 2014;49:966–71.
MacMillan ML, Weisdorf DJ, Wagner JE, DeFor TE, Burns LJ, Ramsay NK, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transpl. 2002;8:387–94.
Bolanos-Meade J, Logan BR, Alousi AM, Antin JH, Barowski K, Carter SL, et al. Phase 3 clinical trial of steroids/mycophenolate mofetil vs steroids/placebo as therapy for acute GVHD: BMT CTN 0802. Blood. 2014;124:3221–7. quiz 3335
Alousi AM, Weisdorf DJ, Logan BR, Bolanos-Meade J, Carter S, Difronzo N, et al. Etanercept, mycophenolate, denileukin, or pentostatin plus corticosteroids for acute graft-versus-host disease: a randomized phase 2 trial from the Blood and Marrow Transplant Clinical Trials Network. Blood. 2009;114:511–7.
Lee SJ, Zahrieh D, Agura E, MacMillan ML, Maziarz RT, McCarthy PL Jr, et al. Effect of up-front daclizumab when combined with steroids for the treatment of acute graft-versus-host disease: results of a randomized trial. Blood. 2004;104:1559–64.
Cahn JY, Bordigoni P, Tiberghien P, Milpied N, Brion A, Widjenes J, et al. Treatment of acute graft-versus-host disease with methylprednisolone and cyclosporine with or without an anti-interleukin-2 receptor monoclonal antibody. A multicenter phase III study. Transplantation. 1995;60:939–42.
Couriel DR, Saliba R, de Lima M, Giralt S, Andersson B, Khouri I, et al. A phase III study of infliximab and corticosteroids for the initial treatment of acute graft-versus-host disease. Biol Blood Marrow Transpl. 2009;15:1555–62.
Cragg L, Blazar BR, Defor T, Kolatker N, Miller W, Kersey J, et al. A randomized trial comparing prednisone with antithymocyte globulin/prednisone as an initial systemic therapy for moderately severe acute graft-versus-host disease. Biol Blood Marrow Transpl. 2000;6:441–7.
Holtan SG, Pasquini M, Weisdorf DJ. Acute graft-versus-host disease: a bench-to-bedside update. Blood. 2014;124:363–73.
Janeway C, Travers P, Walport M. MJS immunobiology: the immune system in health and disease. 5 ed. New York: Garland Science; 2001.
Maus MV, Grupp SA, Porter DL, June CH. Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood. 2014;123:2625–35.
Heeger PS, Lalli PN, Lin F, Valujskikh A, Liu J, Muqim N, et al. Decay-accelerating factor modulates induction of T cell immunity. J Exp Med. 2005;201:1523–30.
Wang Y, Lai P, Chen X, He C, Huang X, Geng S, et al. Attenuation of cGVHD by C5a/C5aR blockade is associated with increased frequency of Treg. Sci Rep. 2017;7:3603.
Chen X, Lai P, Wang Y, He C, Wu S, Huang X, et al. Emerging role of C5a/C5aR IL-17A axis in cGVHD. Am J Transl Res. 2018;10:2148–57.
Von Zabern I, Nolte R, Vogt W. Incompatibility between complement components C3 and C5 of guinea-pig and man, an indication of their interaction in C5 activation by classical and alternative C5 convertases. Scand J Immunol. 1979;9:69–74.
Vogt W, Schmidt G, Von Buttlar B, Dieminger L. A new function of the activated third component of complement: binding to C5, an essential step for C5 activation. Immunology. 1978;34:29–40.
Ma Q, Li D, Carreno R, Patenia R, Tsai KY, Xydes-Smith M, et al. Complement component C3 mediates Th1/Th17 polarization in human T-cell activation and cutaneous GVHD. Bone Marrow Transpl. 2014;49:972–6.
Lalli PN, Strainic MG, Yang M, Lin F, Medof ME, Heeger PS. Locally produced C5a binds to T cell-expressed C5aR to enhance effector T-cell expansion by limiting antigen-induced apoptosis. Blood. 2008;112:1759–66.
Strainic MG, Liu J, Huang D, An F, Lalli PN, Muqim N, et al. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28:425–35.
Cravedi P, Leventhal J, Lakhani P, Ward SC, Donovan MJ, Heeger PS. Immune cell-derived C3a and C5a costimulate human T cell alloimmunity. Am J Transpl. 2013;13:2530–9.
Nguyen H, Kuril S, Bastian D, Kim J, Zhang M, Vaena SG, et al. Complement C3a and C5a receptors promote GVHD by suppressing mitophagy in recipient dendritic cells. JCI Insight. 2018;3:e121697.
Strainic MG, Shevach EM, An F, Lin F, Medof ME. Absence of signaling into CD4(+) cells via C3aR and C5aR enables autoinductive TGF-beta1 signaling and induction of Foxp3(+) regulatory T cells. Nat Immunol. 2013;14:162–71.
Beres AJ, Drobyski WR. The role of regulatory T cells in the biology of graft versus host disease. Front Immunol. 2013;4:163.
Kwan WH, Hashimoto D, Paz-Artal E, Ostrow K, Greter M, Raedler H, et al. Antigen-presenting cell-derived complement modulates graft-versus-host disease. J Clin Invest. 2012;122:2234–8.
Nishimura J-I, DeOliveira D, Chen BJ, Kanakura Y, Rother RP, Chao NJ. Prevention of graft-versus-host disease in mouse model using anti-mouse C5 antibody. Blood. 2007;110:3245.
van der Meij BS, de Graaf P, Wierdsma NJ, Langius JA, Janssen JJ, van Leeuwen PA, et al. Nutritional support in patients with GVHD of the digestive tract: state of the art. Bone Marrow Transpl. 2013;48:474–82.
Papadopoulou A, Lloyd DR, Williams MD, Darbyshire PJ, Booth IW. Gastrointestinal and nutritional sequelae of bone marrow transplantation. Arch Dis Child. 1996;75:208–13.
Fasanmade AA, Adedokun OJ, Olson A, Strauss R, Davis HM. Serum albumin concentration: a predictive factor of infliximab pharmacokinetics and clinical response in patients with ulcerative colitis. Int J Clin Pharm Ther. 2010;48:297–308.
Chaudhury C, Brooks CL, Carter DC, Robinson JM, Anderson CL. Albumin binding to FcRn: distinct from the FcRn-IgG interaction. Biochemistry. 2006;45:4983–90.
MacMillan ML, DeFor TE, Weisdorf DJ. The best endpoint for acute GVHD treatment trials. Blood. 2010;115:5412–7.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–8.
Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow Transpl. 2016;22:4–10.
Hillmen P, Hall C, Marsh JC, Elebute M, Bombara MP, Petro BE, et al. Effect of eculizumab on hemolysis and transfusion requirements in patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2004;350:552–9.
Levine JE, Braun TM, Harris AC, Holler E, Taylor A, Miller H, et al. A prognostic score for acute graft-versus-host disease based on biomarkers: a multicentre study. Lancet Haematol. 2015;2:e21–29.
MacMillan ML, Robin M, Harris AC, DeFor TE, Martin PJ, Alousi A, et al. A refined risk score for acute graft-versus-host disease that predicts response to initial therapy, survival, and transplant-related mortality. Biol Blood Marrow Transpl. 2015;21:761–7.
Mielcarek M, Furlong T, Storer BE, Green ML, McDonald GB, Carpenter PA, et al. Effectiveness and safety of lower dose prednisone for initial treatment of acute graft-versus-host disease: a randomized controlled trial. Haematologica. 2015;100:842–8.
Martin PJ, Schoch G, Fisher L, Byers V, Anasetti C, Appelbaum FR, et al. A retrospective analysis of therapy for acute graft-versus-host disease: initial treatment. Blood. 1990;76:1464–72.
Weisdorf D, Haake R, Blazar B, Miller W, McGlave P, Ramsay N, et al. Treatment of moderate/severe acute graft-versus-host disease after allogeneic bone marrow transplantation: an analysis of clinical risk features and outcome. Blood. 1990;75:1024–30.
Kekre N, Kim HT, Hofer J, Ho VT, Koreth J, Armand P, et al. Phase II trial of natalizumab with corticosteroids as initial treatment of gastrointestinal acute graft-versus-host disease. Bone Marrow Transpl. 2021;56:1006–12.
Cherry MA, Parekh H, Lerner M, Yu Z, Vesely S, Selby G, et al. The role of complement system in graft versus host disease. J Blood Disorders Transfusion. 2015;6:1–7.
Zhang PL, Wilkerson ML, Schworer CM. C4d staining is a valuable marker in identifying chronic GVHD in colonic biopsies following BMT. Bone Marrow Transpl. 2008;42:209–11.
Rubio MT, Durey-Dragon MA, Wang Y, Blouin J, Jacquelin S, Milpied P, et al. Prognostic significance of complement system activation after allogeneic hematopoietic stem cell transplantation. Blood. 2009;114:1166.
Varga L, Poros A, Puskas E, Panya A, Kramer J, Gyodi E, et al. Clinical significance of longitudinal complement measurements in recipients of bone marrow transplant. Bone Marrow Transpl. 1995;15:509–14.
Noel DR, Witherspoon RP, Storb R, Atkinson K, Doney K, Mickelson EM, et al. Does graft-versus-host disease influence the tempo of immunologic recovery after allogeneic human marrow transplantation? An observation on 56 long-term survivors. Blood. 1978;51:1087–105.
Huber-Lang M, Sarma JV, Zetoune FS, Rittirsch D, Neff TA, McGuire SR, et al. Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006;12:682–7.
Burwick RM, Burwick NR, Feinberg BB. Eculizumab fails to inhibit generation of C5a in vivo. Blood. 2014;124:3502–3.
Sugihara T, Kobori A, Imaeda H, Tsujikawa T, Amagase K, Takeuchi K, et al. The increased mucosal mRNA expressions of complement C3 and interleukin-17 in inflammatory bowel disease. Clin Exp Immunol. 2010;160:386–93.
Laufer J, Oren R, Goldberg I, Horwitz A, Kopolovic J, Chowers Y, et al. Cellular localization of complement C3 and C4 transcripts in intestinal specimens from patients with Crohn’s disease. Clin Exp Immunol. 2000;120:30–37.
Halstensen TS, Mollnes TE, Garred P, Fausa O, Brandtzaeg P. Surface epithelium related activation of complement differs in Crohn’s disease and ulcerative colitis. Gut. 1992;33:902–8.
Ahrenstedt O, Knutson L, Nilsson B, Nilsson-Ekdahl K, Odlind B, Hallgren R. Enhanced local production of complement components in the small intestines of patients with Crohn’s disease. N Engl J Med. 1990;322:1345–9.
Halstensen TS, Mollnes TE, Garred P, Fausa O, Brandtzaeg P. Epithelial deposition of immunoglobulin G1 and activated complement (C3b and terminal complement complex) in ulcerative colitis. Gastroenterology. 1990;98:1264–71.
Manthey HD, Woodruff TM, Taylor SM, Monk PN. Complement component 5a (C5a). Int J Biochem Cell Biol. 2009;41:2114–7.
Scola AM, Higginbottom A, Partridge LJ, Reid RC, Woodruff T, Taylor SM, et al. The role of the N-terminal domain of the complement fragment receptor C5L2 in ligand binding. J Biol Chem. 2007;282:3664–71.
Volokhina EB, Bergseth G, van de Kar NC, van den Heuvel LP, Mollnes TE. Eculizumab treatment efficiently prevents C5 cleavage without C5a generation in vivo. Blood. 2015;126:278–9.
Wagner JL, Hugli TE. Radioimmunoassay for anaphylatoxins: a sensitive method for determining complement activation products in biological fluids. Anal Biochem. 1984;136:75–88.
Acknowledgements
The authors would like to thank the investigators from the study sites: Zaid Al-Kadhimi (Emory University Hospital, Atlanta, GA, USA), George Liwei Chen (Roswell Park Cancer Institute, Buffalo, NY, USA), Iskra Pusic (Washington University School of Medicine, St. Louis MO, USA), Michele Donato (John Theurer Cancer Center, Hackensack University Medical Center, Hackensack, NJ, USA), and Stéphane Vigouroux (Centre F. Magendie, Hospital Haut-Leveque, France). The authors thank John Levine, James Ferrara, (Icahn School of Medicine at Mount Sinai, New York, NY), and Susan Abraham (University of Texas MD Anderson Cancer Center, Houston, TX) for contributions to the biomarker analyses. The authors also thank from Alexion Pharmaceuticals: Richard Riese, Susan Faas, and Mittie Doyle for contributions to study design, Kaushik Patra and Fanny O’Brien for extensive review of the data, and Rima Saliba for providing statistical guidance. This study was supported by research funding from Alexion Pharmaceuticals Inc. Medical writing support was provided by Bioscript Group, Macclesfield, UK, funded by Alexion Pharmaceuticals Inc.
Author information
Authors and Affiliations
Contributions
RSM, interpreted the data and wrote the manuscript. BO, helped manage clinical trial as research nurse and reviewed manuscript critically for important intellectual content. AM, KA, YD, BY helped design the study, interpret the data, and reviewed the manuscript. AA, helped design the study, interpreted the data and wrote the manuscript. JYC, ES, SH, HA, RC helped enroll patients, interpret the data, and review the manuscript. VR, SG, GS helped design the study, enrolled patients, interpreted data, and reviewed the manuscript. All authors contributed to the manuscript content, approved the final version of the manuscript and agreed to be accountable for questions related to the accuracy and integrity of the work.
Corresponding author
Ethics declarations
Competing interests
RSM received research funding from CSLBehring, Kadmon and Incyte. HA has received consulting fees from Incyte and BMS. YD, and AM are employees and stockholders of Alexion Pharmaceuticals Inc (now Alexion, Astrazeneca Rare Disease). BY and KA were employees and stockholders of Alexion Pharmaceuticals Inc during the development phase of this manuscript. GS received a research grant from Alexion Pharamceuticals. SH has received consulting fees/honoraria from Incyte, Generon and served participated in a Data Safety Monitoring Board/Advisory Board for CSl Behring. JYC declares receiving honoraria for participating in an Advisory Board for Alexion Pharmaceuticals. BO, VR, SG, RC, and ES declare no competing interests. AA has received research support, and honoraria for participating in an Advisory Board for Alexion Pharmaceuticals.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mehta, R.S., Ali, H., Dai, Y. et al. A prospective phase 2 clinical trial of a C5a complement inhibitor for acute GVHD with lower GI tract involvement. Bone Marrow Transplant 58, 991–999 (2023). https://doi.org/10.1038/s41409-023-01996-4
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41409-023-01996-4
This article is cited by
-
Recent advances in acute gastrointestinal graft versus host disease (aGvHD): aspects of steroid-resistant disease
Annals of Hematology (2025)
-
Hemostasis and complement in allogeneic hematopoietic stem cell transplantation: clinical significance of two interactive systems
Bone Marrow Transplantation (2024)
-
Deposition of complement components C5b-9 and MASP2 in tissues is not a feature of GVHD and may assist in discriminating GVHD from thrombotic microangiopathy following allogenic transplantation
Bone Marrow Transplantation (2023)