Abstract
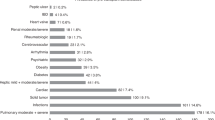
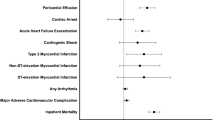
This multicenter study investigates the incidence and predictors of cardiac events (CE) following allo-HCT with PTCY in 453 AML patients. CE occurred in 57 (12.3%) patients within a median of 52 days (IQR: 13–289), with day 100 and 5-year cumulative incidences of 7.7% and 13.5%. Early (first 100 days) and late CE occurred at rates of 7.7% and 4.8%. The most prevalent CE were heart failure (n = 18, 31.6%), pericardial complications (n = 16, 28.1%), and arrhythmia (n = 14, 24.6%). The proportions of patients older than 55 years (64.9% vs. 46.1%, P = 0.010), with hypertension (36.8% vs. 18.4%, P = 0.001) and dyslipidemia (28.1% vs. 11.1%, P = 0.001) were higher in patients with CE. Patients undergoing haplo-HCT trend to have more CE (68.4% vs. 56.8%, P = 0.083). The multivariate regression analysis revealed that only hypertension (HR 1.88, P = 0.036) and dyslipidemia (HR 2.20, P = 0.018) were predictors for CE, with no differences according to donor type (haplo-HCT vs. others: HR 1.33, P = 0.323). Among the 57 patients with CE, the mortality rate was 12.2%. Notably, the diagnosis of CE negatively impacted NRM (HR 2.57, P = 0.011) and OS (HR 1.80, P = 0.009), underscoring necessity of aggressively treating cardiovascular risk factors, and implementing post-transplant cardiac monitoring protocols to prevent these complications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
Data sharing would be only considered after specific request.
References
Lyon AR, Lopez-Fernandez T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J Cardiovasc Imaging. 2022;23:e333–e465.
Braverman AC, Antin JH, Plappert MT, Cook EF, Lee RT. Cyclophosphamide cardiotoxicity in bone marrow transplantation: a prospective evaluation of new dosing regimens. J Clin Oncol. 1991;9:1215–23.
Ishida S, Doki N, Shingai N, Yoshioka K, Kakihana K, Sakamaki H, et al. The clinical features of fatal cyclophosphamide-induced cardiotoxicity in a conditioning regimen for allogeneic hematopoietic stem cell transplantation (allo-HSCT). Ann Hematol. 2016;95:1145–50.
Marumo A, Omori I, Tara S, Otsuka Y, Konuma R, Adachi H, et al. Cyclophosphamide-induced cardiotoxicity at conditioning for allogeneic hematopoietic stem cell transplantation would occur among the patients treated with 120 mg/kg or less. Asia Pac J Clin Oncol. 2022;18:e507–e14.
Rotz S, Hamilton BK. Post-transplantation cyclophosphamide: an old nemesis to a new transplant paradigm?. JACC CardioOncol. 2021;3:260–2.
Dulery R, Mohty R, Labopin M, Sestili S, Malard F, Brissot E, et al. Early cardiac toxicity associated with post-transplant cyclophosphamide in allogeneic stem cell transplantation. JACC CardioOncol. 2021;3:250–9.
Yeh J, Whited L, Saliba RM, Rondon G, Banchs J, Shpall E, et al. Cardiac toxicity after matched allogeneic hematopoietic cell transplant in the posttransplant cyclophosphamide era. Blood Adv. 2021;5:5599–607.
Perez-Valencia AI, Cascos E, Carbonell-Ordeig S, Charry P, Gomez-Hernando M, Rodriguez-Lobato LG, et al. Incidence, risk factors, and impact of early cardiac toxicity after allogeneic hematopoietic cell transplant. Blood Adv. 2023;7:2018–31.
Lin CJ, Vader JM, Slade M, DiPersio JF, Westervelt P, Romee R. Cardiomyopathy in patients after posttransplant cyclophosphamide-based hematopoietic cell transplantation. Cancer. 2017;123:1800–9.
Snowden JA, Sanchez-Ortega I, Corbacioglu S, Basak GW, Chabannon C, de la Camara R, et al. Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2022. Bone Marrow Transpl. 2022;57:1217–39.
Kanate AS, Majhail NS, Savani BN, Bredeson C, Champlin RE, Crawford S, et al. Indications for hematopoietic cell transplantation and immune effector cell therapy: guidelines from the American Society for transplantation and cellular therapy. Biol Blood Marrow Transpl. 2020;26:1247–56.
Schoemans HM, Lee SJ, Ferrara JL, Wolff D, Levine JE, Schultz KR, et al. EBMT-NIH-CIBMTR task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone Marrow Transpl. 2018;53:1401–15.
Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the mount sinai acute GVHD international consortium. Biol Blood Marrow Transpl. 2016;22:4–10.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 diagnosis and staging working group report. Biol Blood Marrow Transpl. 2015;21:389–401.e1.
Sureda A, Carpenter PA, Bacigalupo A, Bhatt VR, de la Fuente J, Ho A, et al. Harmonizing definitions for hematopoietic recovery, graft rejection, graft failure, poor graft function, and donor chimerism in allogeneic hematopoietic cell transplantation: a report on behalf of the EBMT, ASTCT, CIBMTR, and APBMT. Bone Marrow Transpl. 59:832–837.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013;48:452–8.
Tichelli A, Bhatia S, Socie G. Cardiac and cardiovascular consequences after haematopoietic stem cell transplantation. Br J Haematol. 2008;142:11–26.
Tichelli A, Passweg J, Wojcik D, Rovo A, Harousseau JL, Masszi T, et al. Late cardiovascular events after allogeneic hematopoietic stem cell transplantation: a retrospective multicenter study of the Late Effects Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2008;93:1203–10.
Iqubal A, Iqubal MK, Sharma S, Ansari MA, Najmi AK, Ali SM, et al. Molecular mechanism involved in cyclophosphamide-induced cardiotoxicity: Old drug with a new vision. Life Sci. 2019;218:112–31.
Nishikawa T, Miyahara E, Kurauchi K, Watanabe E, Ikawa K, Asaba K, et al. Mechanisms of fatal cardiotoxicity following high-dose cyclophosphamide therapy and a method for its prevention. PLoS One. 2015;10:e0131394.
Tolosa-Ridao C, Cascos E, Rodriguez-Lobato LG, Pedraza A, Suarez-Lledo M, Charry P, et al. EASIX and cardiac adverse events after allogeneic hematopoietic cell transplantation. Bone Marrow Transpl. 2024.59:974–982
Martinez-Sanchez J, Pascual-Diaz R, Palomo M, Moreno-Castano AB, Ventosa H, Salas MQ, et al. Mafosfamide, a cyclophosphamide analog, causes a proinflammatory response and increased permeability on endothelial cells in vitro. Bone Marrow Transpl. 2023;58:407–13.
Moreno-Castano AB, Salas MQ, Palomo M, Martinez-Sanchez J, Rovira M, Fernandez-Aviles F, et al. Early vascular endothelial complications after hematopoietic cell transplantation: Role of the endotheliopathy in biomarkers and target therapies development. Front Immunol. 2022;13:1050994.
Dulery R, Malard F, Brissot E, Banet A, Sestili S, Belhocine R, et al. Reduced post-transplant cyclophosphamide dose with antithymocyte globulin in peripheral blood stem cell haploidentical transplantation. Bone Marrow Transpl. 2023;58:1215–22.
Zhang W, Gui R, Zu Y, Zhang B, Li Z, Zhang Y, et al. Reduced-dose post-transplant cyclophosphamide plus low-dose post-transplant anti-thymocyte globulin as graft-versus-host disease prophylaxis with fludarabine-busulfan-cytarabine conditioning in haploidentical peripheral blood stem cell transplantation: A multicentre, randomized controlled clinical trial. Br J Haematol. 2023;200:210–21.
Zu Y, Li Z, Gui R, Liu Y, Zhang Y, Yu F, et al. Low-dose post-transplant cyclophosphamide with low-dose antithymocyte globulin for prevention of graft-versus-host disease in first complete remission undergoing 10/10 HLA-matched unrelated donor peripheral blood stem cell transplants: a multicentre, randomized controlled trial. Bone Marrow Transpl. 2022;57:1573–80.
Garcia-Cadenas I, Redondo S, Esquirol A, Portos JM, Novelli S, Saavedra S, et al. Successful outcome in patients with myelofibrosis undergoing allogeneic donor hematopoietic cell transplantation using reduced doses of post-transplantation cyclophosphamide: challenges and review of the literature. Transpl Cell Ther. 2023;29:473.e1–e6.
Yanagisawa R, Tamaki M, Tanoshima R, Misaki Y, Uchida N, Koi S, et al. Risk factors for fatal cardiac complications after allogeneic hematopoietic cell transplantation: Japanese Society for Transplantation and Cellular Therapy transplant complications working group. Hematol Oncol. 2023;41:535–45.
Kalam K, Marwick TH. Role of cardioprotective therapy for prevention of cardiotoxicity with chemotherapy: a systematic review and meta-analysis. Eur J Cancer. 2013;49:2900–9.
Li X, Li Y, Zhang T, Xiong X, Liu N, Pang B, et al. Role of cardioprotective agents on chemotherapy-induced heart failure: a systematic review and network meta-analysis of randomized controlled trials. Pharm Res. 2020;151:104577.
Bolanos-Meade J, Fuchs EJ, Luznik L, Lanzkron SM, Gamper CJ, Jones RJ, et al. HLA-haploidentical bone marrow transplantation with posttransplant cyclophosphamide expands the donor pool for patients with sickle cell disease. Blood. 2012;120:4285–91.
Broers AEC, de Jong CN, Bakunina K, Hazenberg MD, van Marwijk Kooy M, de Groot MR, et al. Posttransplant cyclophosphamide for prevention of graft-versus-host disease: results of the prospective randomized HOVON-96 trial. Blood Adv. 2022;6:3378–85.
Garcia-Cadenas I, Awol R, Esquirol A, Saavedra S, Bosch-Vilaseca A, Novelli S, et al. Incorporating posttransplant cyclophosphamide-based prophylaxis as standard-of-care outside the haploidentical setting: challenges and review of the literature. Bone Marrow Transpl. 2020;55:1041–9.
Ruggeri A, Labopin M, Bacigalupo A, Afanasyev B, Cornelissen JJ, Elmaagacli A, et al. Post-transplant cyclophosphamide for graft-versus-host disease prophylaxis in HLA matched sibling or matched unrelated donor transplant for patients with acute leukemia, on behalf of ALWP-EBMT. J Hematol Oncol. 2018;11:40.
Acknowledgements
We thank our patients and the nursing and support staff in the Hematopoietic Cell Transplant Programs and the Cardiology Departments participating in the study and the support provided by the Grupo Español de Trasplante de Progenitores Hematopoyéticos y Terapia Celular. We additionally thank REDCap (Research Electronic Data Capture) service for permitting the use of their service without costs. REDCap is a secure, web-based software platform designed to support data capture for research studies.
Author information
Authors and Affiliations
Contributions
MQS and EC designed the study, conducted the statistical analysis, and drafted the paper. The GETH-TC facilitated and endorsed the study’s execution. Dr. MJC provided valuable insights into the study’s execution and interpretation of results. All coinvestigators involved in the study have diligently collected the data and supported the integration of the results in the present manuscript. ALG, EP, MBG, LLC, MJPC, ML, AE, FPM, IHF, IQQ, AJSM, SFL, SVF, MFSG, LGP, APG, TT, LC, SF, AC, PB, GO and MJC contributed significantly to the study, offering valuable feedback, and critically reviewing and approving the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Salas, M.Q., Cascos, E., López-García, A. et al. Cardiac events occurring after allogeneic hematopoietic cell transplantation with post-transplant cyclophosphamide. Study conducted on behalf of the GETH-TC. Bone Marrow Transplant 59, 1694–1703 (2024). https://doi.org/10.1038/s41409-024-02414-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41409-024-02414-z
This article is cited by
-
Posttransplant cyclophosphamide versus antithymocyte globulin in patients with cardiovascular comorbidity undergoing allogeneic hematopoietic cell transplantation for acute myeloid leukaemia in first complete remission from unrelated donors: a retrospective matched-pair analysis from the ALWP of the EBMT
Bone Marrow Transplantation (2025)