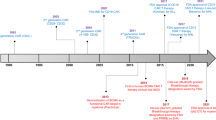
Abstract
The CAR-T cell products ciltacabtagene autoleucel and idecabtagene vicleucel have transformed the management of patients with multiple myeloma. Here, we present a practical guide highlighting clinical pearls on the incorporation of CAR-T into clinical practice. Topics addressed include expected outcomes, recommendations for referral timing, bridging therapy, treatment complications, therapeutic sequencing, and management of relapse.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Sheykhhasan M, Ahmadieh-Yazdi A, Vicidomini R, Poondla N, Tanzadehpanah H, Dirbaziyan A, et al. CAR T therapies in multiple myeloma: unleashing the future. Cancer Gene Ther. 2024;31:667–86.
National Comprehenisve Cancer Network. NCCN Guidelines: Multiple Myeloma, Version 4.2024. 2024 [cited 2024 Jul 13]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf
Research C for DE and. FDA approves idecabtagene vicleucel for multiple myeloma. FDA. 2021 Jun 11 [cited 2024 Jul 13]; Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-idecabtagene-vicleucel-multiple-myeloma
Munshi NC, Anderson LD, Shah N, Madduri D, Berdeja J, Lonial S, et al. Idecabtagene Vicleucel in relapsed and Refractory multiple Myeloma. N. Engl J Med. 2021;384:705–16.
Berdeja JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398:314–24.
Cohen AD, Parekh S, Santomasso BD, Gállego Pérez-Larraya J, van de Donk NWCJ, Arnulf B, et al. Incidence and management of CAR-T neurotoxicity in patients with multiple myeloma treated with ciltacabtagene autoleucel in CARTITUDE studies. Blood Cancer J. 2022;12:1–9.
Ailawadhi S, Shune L, Wong SW, Lin Y, Patel K, Jagannath S. Optimizing the CAR T-cell therapy experience in multiple myeloma: clinical pearls from an expert roundtable. Clin Lymphoma, Myeloma Leuk. 2024;24:e217–25.
Villanueva R, Hansen DK, Tonseth RP, Gage KL, Wei Z, De Avila G, et al. High metabolic tumor volume is associated with higher toxicity and decreased efficacy of BCMA CAR-T cell therapy in multiple myeloma. Blood. 2022;140:10402–4.
Baguet C, Larghero J, Mebarki M. Early predictive factors of failure in autologous CAR T-cell manufacturing and/or efficacy in hematologic malignancies. Blood Adv. 2024;8:337–42.
Jo T, Yoshihara S, Okuyama Y, Fujii K, Henzan T, Kahata K, et al. Risk factors for CAR-T cell manufacturing failure among DLBCL patients: A nationwide survey in Japan. Br J Haematol. 2023;202:256–66.
Cancer Network [Internet]. 2024 [cited 2024 Jul 13]. FDA Approves Ide-Cel in Previously Treated Multiple Myeloma. Available from: https://www.cancernetwork.com/view/fda-approves-ide-cel-in-previously-treated-multiple-myeloma
Research C for BE and. CARVYKTI. FDA [Internet]. 2024 Jun 13 [cited 2024 Jul 14]; Available from: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/carvykti
Package Insert: ABECMA [Internet]. [cited 2024 Jul 13]. Available from: https://www.fda.gov/media/147055/download?attachment
Package Insert: CARVYKTI [Internet]. [cited 2024 Jul 13]. Available from: https://www.fda.gov/media/156560/download
Anderson LD, Dhakal B, Jain T, Oluwole OO, Shah GL, Sidana S, et al. Chimeric Antigen Receptor T cell therapy for myeloma: where are we now and what is needed to move chimeric antigen receptor T cells forward to earlier lines of therapy? Expert Panel Opinion from the American Society for Transplantation and Cellular Therapy. Transplant Cell Ther, Off Publ Am Soc Transplant Cell Ther. 2024;30:17–37.
Mitra A, Barua A, Huang L, Ganguly S, Feng Q, He B From bench to bedside: the history and progress of CAR T cell therapy. Front Immunol. 2023 May 15 [cited 2024 Jul 14];14. Available from: https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2023.1188049/full
Jain MD, Smith M, Shah NN. How I treat refractory CRS and ICANS after CAR T-cell therapy. Blood. 2023;141:2430–42.
Jain T, Olson TS, Locke FL. How I treat cytopenias after CAR T-cell therapy. Blood. 2023;141:2460–9.
Gutierrez C, Neilan TG, Grover NS. How I approach optimization of patients at risk of cardiac and pulmonary complications after CAR T-cell therapy. Blood. 2023;141:2452–9.
Geethakumari PR, Ramasamy DP, Dholaria B, Berdeja J, Kansagra A. Balancing quality, cost, and access during delivery of newer cellular and immunotherapy treatments. Curr Hematol Malig Rep. 2021;16:345–56.
Hansen DK, Sidana S, Peres LC, Colin Leitzinger C, Shune L, Shrewsbury A, et al. Idecabtagene Vicleucel for relapsed/refractory multiple myeloma: real-world experience from the myeloma CAR T Consortium. J Clin Oncol. 2023;41:2087–97.
Sidana S, Patel KK, Peres LC, Bansal R, Kocoglu MH, Shune L, et al. Safety and efficacy of standard-of-care ciltacabtagene autoleucel for relapsed/refractory multiple myeloma. Blood. 2025;145:85–97.
Santomasso BD, Gust J, Perna F. How I treat unique and difficult-to-manage cases of CAR T-cell therapy–associated neurotoxicity. Blood. 2023;141:2443–51.
Bhaskar ST, Dholaria BR, Sengsayadeth SM, Savani BN, Oluwole OO. Role of bridging therapy during chimeric antigen receptor T cell therapy. EJHaem. 2021;3:39–45.
St Martin Y, Franz JK, Agha ME, Lazarus HM. Failure of CAR-T cell therapy in relapsed and refractory large cell lymphoma and multiple myeloma: An urgent unmet need. Blood Rev. 2023;60:101095.
Levine BL, Miskin J, Wonnacott K, Keir C. Global manufacturing of CAR T cell therapy. Mol Ther Methods Clin Dev. 2017;4:92–101.
Ayala Ceja M, Khericha M, Harris CM, Puig-Saus C, Chen YY. CAR-T cell manufacturing: Major process parameters and next-generation strategies. J Exp Med. 2024;221:e20230903.
Al Hadidi S, Szabo A, Esselmann J, Hammons L, Hussain M, Ogunsesan Y, et al. Clinical outcome of patients with relapsed refractory multiple myeloma listed for BCMA directed commercial CAR-T therapy. Bone Marrow Transpl. 2023;58:443–5.
Kourelis T, Bansal R, Berdeja J, Siegel D, Patel K, Mailankody S, et al. Ethical challenges with multiple myeloma BCMA chimeric antigen receptor T cell slot allocation: a multi-institution experience. Transpl Cell Ther. 2023;29:255–8.
Bowden B, Ciccone D, Salmon J, Alegria V, Kallenbach L, De Braganca KC. Using real-world remanufacturing and recollection data to optimize time-to-treatment in patients with out-of-specification Ciltacabtagene Autoleucel. Blood. 2023;142:6918.
Firestone RS, Mailankody S. Current use of CAR T cells to treat multiple myeloma. Hematology. 2023;2023:340–7.
Rodriguez-Otero P, Ailawadhi S, Arnulf B, Patel K, Cavo M, Nooka AK, et al. Ide-cel or standard regimens in relapsed and refractory multiple myeloma. N Engl J Med. 2023;388:1002–14.
Rodriguez-Otero P, Usmani S, Cohen AD, van de Donk NWCJ, Leleu X, Gállego Pérez-Larraya J, et al. International Myeloma Working Group immunotherapy committee consensus guidelines and recommendations for optimal use of T-cell-engaging bispecific antibodies in multiple myeloma. Lancet Oncol. 2024;25:e205–16.
Dhakal B, Akhtar OS, Cowan AJ, Richard S, Friend R, Rees MJ, et al. Talquetamab bridging: Paving the Way to B-cell maturation Antigen (BCMA) CAR-T Cell Therapy in Relapsed/Refractory Multiple Myeloma (RRMM). Blood. 2024;144:931.
Roddie C, Neill L, Osborne W, Iyengar S, Tholouli E, Irvine D, et al. Effective bridging therapy can improve CD19 CAR-T outcomes while maintaining safety in patients with large B-cell lymphoma. Blood Adv. 2023;7:2872–83.
Shahid S, Ramaswamy K, Flynn J, Mauguen A, Perica K, Park JH, et al. Impact of bridging chemotherapy on clinical outcomes of CD19-Specific CAR T cell therapy in children/young adults with relapsed/refractory B cell acute lymphoblastic leukemia. Transpl Cell Ther. 2022;28:72.e1–72.e8.
Costa BA, Flynn J, Nishimura N, Devlin SM, Farzana T, Rajeeve S, et al. Prognostic impact of corticosteroid and tocilizumab use following chimeric antigen receptor T-cell therapy for multiple myeloma. Blood Cancer J. 2024;14:84.
Hines MR, Knight TE, McNerney KO, Leick MB, Jain T, Ahmed S, et al. Immune Effector Cell-Associated Hemophagocytic Lymphohistiocytosis-Like Syndrome. Transplant Cell Ther. 2023;29:438.e1–438.e16.
Wohlfarth P, Agis H, Gualdoni GA, Weber J, Staudinger T, Schellongowski P, et al. Interleukin 1 Receptor Antagonist Anakinra, Intravenous Immunoglobulin, and Corticosteroids in the Management of Critically Ill Adult Patients With Hemophagocytic Lymphohistiocytosis. J Intensive Care Med. 2019;34:723–31.
Wang J, Wang Y, Wu L, Wang X, Jin Z, Gao Z, et al. Ruxolitinib for refractory/relapsed hemophagocytic lymphohistiocytosis. Haematologica. 2020;105:e210–2.
Wang J, Zhang R, Wu X, Li F, Yang H, Liu L, et al. Ruxolitinib-combined doxorubicin-etoposide-methylprednisolone regimen as a salvage therapy for refractory/relapsed haemophagocytic lymphohistiocytosis: a single-arm, multicentre, phase 2 trial. Br J Haematol. 2021;193:761–8.
Fortuna GG, Banerjee R, Savid-Frontera C, Song J, Morán-Segura CM, Nguyen JV, et al. Immune effector cell-associated enterocolitis following chimeric antigen receptor T-cell therapy in multiple myeloma. Blood Cancer J. 2024;14:1–8.
ONS 2024: Incidence and Management of Cranial Nerve Impairments in Patients With Multiple Myeloma Treated With Ciltacabtagene Autoleucel in CARTITUDE Studies [Internet]. [cited 2025 Jan 22]. Available from: https://clin.larvol.com/abstract-detail/ONS%202024/70145524
Karschnia P, Miller KC, Yee AJ, Rejeski K, Johnson PC, Raje N, et al. Neurologic toxicities following adoptive immunotherapy with BCMA-directed CAR T cells. Blood. 2023;142:1243–8.
Van Oekelen O, Aleman A, Upadhyaya B, Schnakenberg S, Madduri D, Gavane S, et al. Neurocognitive and hypokinetic movement disorder with features of parkinsonism after BCMA-targeting CAR-T cell therapy. Nat Med. 2021;27:2099–103.
Mejia Saldarriaga M, Pan D, Unkenholz C, Mouhieddine TH, Velez-Hernandez JE, Engles K, et al. Absolute lymphocyte count after BCMA CAR-T therapy is a predictor of response and outcomes in relapsed multiple myeloma. Blood Adv. 2024;8:3859–69.
Rejeski K, Subklewe M, Aljurf M, Bachy E, Balduzzi A, Barba P, et al. Immune effector cell–associated hematotoxicity: EHA/EBMT consensus grading and best practice recommendations. Blood. 2023;142:865–77.
Rejeski K, Perez A, Sesques P, Hoster E, Berger C, Jentzsch L, et al. CAR-HEMATOTOX: a model for CAR T-cell–related hematologic toxicity in relapsed/refractory large B-cell lymphoma. Blood. 2021;138:2499–513.
Rejeski K, Hansen DK, Bansal R, Sesques P, Ailawadhi S, Logue JM, et al. The CAR-HEMATOTOX score as a prognostic model of toxicity and response in patients receiving BCMA-directed CAR-T for relapsed/refractory multiple myeloma. J Hematol Oncol. 2023;16:88.
Davis JA, Sborov DW, Wesson W, Julian K, Abdallah AO, McGuirk JP, et al. Efficacy and Safety of CD34+ Stem Cell Boost for Delayed Hematopoietic Recovery After BCMA Directed CAR T-cell Therapy. Transplant Cell Ther. 2023;29:567–71.
Wat J, Barmettler S. Hypogammaglobulinemia after Chimeric Antigen Receptor (CAR) T-cell therapy: characteristics, management, and future directions. J Allergy Clin Immunol Pr. 2022;10:460–6.
Wang Y, Li C, Xia J, Li P, Cao J, Pan B, et al. Humoral immune reconstitution after anti-BCMA CAR T-cell therapy in relapsed/refractory multiple myeloma. Blood Adv. 2021;5:5290–9.
Lancman G, Parsa K, Kotlarz K, Avery L, Lurie A, Lieberman-Cribbin A, et al. IVIg Use Associated with Ten-Fold Reduction of Serious Infections in Multiple Myeloma Patients Treated with Anti-BCMA Bispecific Antibodies. Blood Cancer Discov. 2023;4:440–51.
Ferreri CJ, Hildebrandt MAT, Hashmi H, Shune LO, McGuirk JP, Sborov DW, et al. Real-world experience of patients with multiple myeloma receiving ide-cel after a prior BCMA-targeted therapy. Blood Cancer J. 2023;13:1–9.
Charting the Course: Sequencing Immunotherapy for Multiple Myeloma | American Society of Clinical Oncology Educational Book [Internet]. [cited 2024 Jul 14]. Available from: https://ascopubs.org/doi/10.1200/EDBK_432204
Samur MK, Fulciniti M, Aktas Samur A, Bazarbachi AH, Tai YT, Prabhala R, et al. Biallelic loss of BCMA as a resistance mechanism to CAR T cell therapy in a patient with multiple myeloma. Nat Commun. 2021;12:868.
Li W, Zhang B, Cao W, Zhang W, Li T, Liu L, et al. Identification of potential resistance mechanisms and therapeutic targets for the relapse of BCMA CAR-T therapy in relapsed/refractory multiple myeloma through single-cell sequencing. Exp Hematol Oncol. 2023;12:44.
Patel U, Oluwole OO, Kassim A, Jayani R, Belliveau P, Savani B, et al. Sequencing bispecific antibodies and CAR T cell therapy in multiple myeloma with prior exposure to BCMA-targeted therapies. JCO. 2023;41:e20049–e20049.
Funding
Citiius, Gamida cell, Incyte. Advisory Board Membership: Abvie, CRISPR, Allogene, Astra Zeneca, BeiGene.
Author information
Authors and Affiliations
Contributions
DO researched and wrote the manuscript. VB edited and helped write the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
O’Leary, D., Bachanova, V. CAR-T for multiple myeloma: practice pearls. Bone Marrow Transplant 60, 940–947 (2025). https://doi.org/10.1038/s41409-025-02582-6
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41409-025-02582-6