Abstract
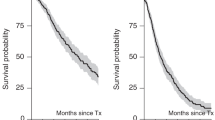
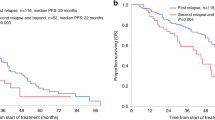
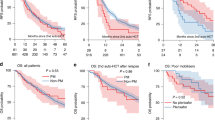
Autologous hematopoietic cell transplantation (autoHCT) remains a therapeutic option for multiple myeloma (MM) at relapse. We retrospectively analyzed 650 patients who underwent delayed (n = 335) or salvage (n = 315) autoHCT at a single center from 2006–2023. Median age was 61.4 years; 22% were Black, and 21% had high-risk cytogenetics. Forty-nine percent received >3 prior therapy lines, and 33% were lenalidomide-refractory. Non-relapse mortality was 3% at day 100 and 4% at 1 year. Median progression-free survival (mPFS) was 17.5 months and median overall survival (mOS) 47.3 months, with no significant difference between delayed and salvage autoHCT (mPFS 16.3 vs. 19.1 months; mOS 43.2 vs. 50.8 months). In salvage autoHCT, transplant ≥24 months after first autoHCT was associated with superior outcomes (mPFS 20.6 vs. 8.4 months; mOS 54.6 vs. 12.5 months; p < 0.001). Multivariable analysis identified adverse factors for PFS and OS including high-risk cytogenetics, R-ISS stage II–III, lenalidomide- or carfilzomib-refractory disease, anti-CD38 antibody non-exposure, and >3 prior therapy lines; achieving CR post-transplant and receiving maintenance predicted improved outcomes. This largest single-center cohort demonstrates delayed or salvage autoHCT is feasible and effective, particularly for patients with prolonged first remissions, and provides a benchmark for emerging therapies in relapsed/refractory MM.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
Richardson PG, Jacobus SJ, Weller EA, Hassoun H, Lonial S, Raje NS, et al. Triplet Therapy, Transplantation, and Maintenance until Progression in Myeloma. N Engl J Med. 2022;387:132–47.
Perrot A, Lauwers-Cances V, Cazaubiel T, Facon T, Caillot D, Clement-Filliatre L, et al. Early Versus Late Autologous Stem Cell Transplant in Newly Diagnosed Multiple Myeloma: Long-Term Follow-up Analysis of the IFM 2009 Trial. Blood. 2020;136:39.
Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. N Engl J Med. 2017;376:1311–20.
Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Français du Myélome N Engl J Med. 1996;335:91–7.
Sonneveld P, Dimopoulos MA, Boccadoro M, Quach H, Ho PJ, Beksac M, et al. Daratumumab, Bortezomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl J Med. 2024;390:301–13.
Facon T, Dimopoulos MA, Leleu XP, Beksac M, Pour L, Hájek R, et al. Isatuximab, Bortezomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl J Med. 2024;391:1597–609.
San-Miguel J, Dhakal B, Yong K, Spencer A, Anguille S, Mateos MV, et al. Cilta-cel or Standard Care in Lenalidomide-Refractory Multiple Myeloma. N Engl J Med. 2023;389:335–47.
Rodriguez-Otero P, Ailawadhi S, Arnulf B, Patel K, Cavo M, Nooka AK, et al. Ide-cel or Standard Regimens in Relapsed and Refractory Multiple Myeloma. N Engl J Med. 2023;388:1002–14.
Moreau P, Garfall AL, van de Donk N, Nahi H, San-Miguel JF, Oriol A, et al. Teclistamab in Relapsed or Refractory Multiple Myeloma. N Engl J Med. 2022;387:495–505.
Lesokhin AM, Tomasson MH, Arnulf B, Bahlis NJ, Miles Prince H, Niesvizky R, et al. Elranatamab in relapsed or refractory multiple myeloma: phase 2 MagnetisMM-3 trial results. Nat Med. 2023;29:2259–67.
Chari A, Minnema MC, Berdeja JG, Oriol A, van de Donk N, Rodríguez-Otero P, et al. Talquetamab, a T-Cell-Redirecting GPRC5D Bispecific Antibody for Multiple Myeloma. N Engl J Med. 2022;387:2232–44.
Pasvolsky O, Marcoux C, Dai J, Milton DR, Tanner MR, Syed N, et al. Trends in outcomes after upfront autologous transplant for multiple myeloma over three decades. Transpl Cell Ther. 2024;30:772.e1-772.
Chakraborty R, Hamilton BK, Hashmi SK, Kumar SK, Majhail NS. Health-Related Quality of Life after Autologous Stem Cell Transplantation for Multiple Myeloma. Biol Blood Marrow Transpl. 2018;24:1546–53.
Rajkumar SV. Multiple myeloma: 2024 update on diagnosis, risk-stratification, and management. Am J Hematol. 2024;99:1802–24.
Manjappa S, Fiala MA, King J, Kohnen DA, Vij R. The efficacy of salvage autologous stem cell transplant among patients with multiple myeloma who received maintenance therapy post initial transplant. Bone Marrow Transpl. 2018;53:1483–6.
Lemieux C, Muffly LS, Iberri DJ, Craig JK, Johnston LJ, Lowsky R, et al. Outcomes after delayed and second autologous stem cell transplant in patients with relapsed multiple myeloma. Bone Marrow Transpl. 2021;56:2664–71.
Klomberg KM, Gelderloos M, Kooistra HAM, Nijland M, Huls GA, Roeloffzen WWH, et al. Consolidation With Second High Dose Therapy and Autologous Stem Cell Transplantation Is Associated With Improved Overall Survival in Patients With Multiple Myeloma in First Relapse. Clin Lymphoma Myeloma Leuk. 2025;25:357–64.e5.
Dhakal B, D’Souza A, Kleman A, Chhabra S, Mohan M, Hari P. Salvage second transplantation in relapsed multiple myeloma. Leukemia. 2021;35:1214–7.
Drozd-Sokołowska J, Gras L, Zinger N, Snowden JA, Arat M, Basak G, et al. Autologous hematopoietic cell transplantation for relapsed multiple myeloma performed with cells procured after previous transplantation-study on behalf of CMWP of the EBMT. Bone Marrow Transpl. 2022;57:633–40.
Cook G, Ashcroft AJ, Cairns DA, Williams CD, Brown JM, Cavenagh JD, et al. The effect of salvage autologous stem-cell transplantation on overall survival in patients with relapsed multiple myeloma (final results from BSBMT/UKMF Myeloma X Relapse [Intensive]): a randomised, open-label, phase 3 trial. Lancet Haematol. 2016;3:e340–51.
Baertsch MA, Schlenzka J, Hielscher T, Raab MS, Sauer S, Merz M, et al. Salvage autologous transplant in relapsed multiple myeloma: long-term follow-up of the phase 3 GMMG ReLApsE trial. Blood. 2025;145:1780–7.
Goldschmidt H, Baertsch MA, Schlenzka J, Becker N, Habermehl C, Hielscher T, et al. Salvage autologous transplant and lenalidomide maintenance vs. lenalidomide/dexamethasone for relapsed multiple myeloma: the randomized GMMG phase III trial ReLApsE. Leukemia. 2021;35:1134–44.
Khan S, Reece D, Atenafu EG, Bhella S, Chen C, Masih-Khan E, et al. Post Salvage Therapy Autologous Transplant for Relapsed Myeloma, Ongoing Relevance within Modern Treatment Paradigms? Clin Lymphoma Myeloma Leuk. 2023;23:e97–e106.
Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–e46.
Dimopoulos MA, Moreau P, Terpos E, Mateos MV, Zweegman S, Cook G, et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32:309–22.
Mikhael J, Ismaila N, Cheung MC, Costello C, Dhodapkar MV, Kumar S, et al. Treatment of Multiple Myeloma: ASCO and CCO Joint Clinical Practice Guideline. J Clin Oncol. 2019;37:1228–63.
Pasvolsky O, Yeshurun M, Fraser R, Estrada-Merly N, Rozovski U, Shargian-Alon L, et al. Maintenance therapy after second autologous hematopoietic cell transplantation for multiple myeloma. A CIBMTR analysis. Bone Marrow Transpl. 2022;57:31–7.
Grövdal M, Nahi H, Gahrton G, Liwing J, Waage A, Abildgaard N, et al. Autologous stem cell transplantation versus novel drugs or conventional chemotherapy for patients with relapsed multiple myeloma after previous ASCT. Bone Marrow Transpl. 2015;50:808–12.
Siegel DS, Dimopoulos MA, Ludwig H, Facon T, Goldschmidt H, Jakubowiak A, et al. Improvement in Overall Survival With Carfilzomib, Lenalidomide, and Dexamethasone in Patients With Relapsed or Refractory Multiple Myeloma. J Clin Oncol. 2018;36:728–34.
Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, et al. Overall Survival With Daratumumab, Lenalidomide, and Dexamethasone in Previously Treated Multiple Myeloma (POLLUX): A Randomized, Open-Label, Phase III Trial. J Clin Oncol. 2023;41:1590–9.
Usmani SZ, Quach H, Mateos MV, Landgren O, Leleu X, Siegel D, et al. Final analysis of carfilzomib, dexamethasone, and daratumumab vs carfilzomib and dexamethasone in the CANDOR study. Blood Adv. 2023;7:3739–48.
Yong K, Martin T, Dimopoulos MA, Mikhael J, Capra M, Facon T, et al. Isatuximab plus carfilzomib-dexamethasone versus carfilzomib-dexamethasone in patients with relapsed multiple myeloma (IKEMA): overall survival analysis of a phase 3, randomised, controlled trial. Lancet Haematol. 2024;11:e741–e50.
Jagannath S, Martin TG, Lin Y, Cohen AD, Raje N, Htut M, et al. Long-Term (≥5-Year) Remission and Survival After Treatment With Ciltacabtagene Autoleucel in CARTITUDE-1 patients with relapsed/refractory multiple myeloma. J Clin Oncol. 2025;43:2766–71.
Gagelmann N, Garderet L, Iacobelli S, Koster L, Carotti A, Schönland S, et al. Salvage Transplant Versus CAR-T Cell Therapy for Relapsed Multiple Myeloma. Blood. 2023;142:3592.
Berdeja JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398:314–24.
Sidana S, Patel KK, Peres LC, Bansal R, Kocoglu MH, Shune L, et al. Safety and efficacy of standard-of-care ciltacabtagene autoleucel for relapsed/refractory multiple myeloma. Blood. 2025;145:85–97.
Cordas Dos Santos DM, Tix T, Shouval R, Gafter-Gvili A, Alberge JB, Cliff ERS, et al. A systematic review and meta-analysis of nonrelapse mortality after CAR T cell therapy. Nat Med. 2024;30:2667–78.
Tomasson MH, Iida S, Niesvizky R, Mohty M, Bahlis NJ, Martinez-Lopez J, et al. Long-term survival and safety of elranatamab in patients with relapsed or refractory multiple myeloma: Update from the MagnetisMM-3 study. Hemasphere. 2024;8:e136.
Schinke, Touzeau CD, Minnema C, Donk MC, NWCJvd, Rodríguez-Otero P, et al. Pivotal phase 2 MonumenTAL-1 results of talquetamab (tal), a GPRC5DxCD3 bispecific antibody (BsAb), for relapsed/refractory multiple myeloma (RRMM). J Clin Oncol. 2023;41:8036.
Frenking JH, Riedhammer C, Teipel R, Bassermann F, Besemer B, Bewarder M, et al. A German multicenter real-world analysis of talquetamab in 138 patients with relapsed/refractory multiple myeloma. Hemasphere. 2025;9:e70114.
Riedhammer C, Bassermann F, Besemer B, Bewarder M, Brunner F, Carpinteiro A, et al. Real-world analysis of teclistamab in 123 RRMM patients from Germany. Leukemia. 2024;38:365–71.
Malard F, Bobin A, Labopin M, Karlin L, Frenzel L, Roussel M, et al. Elranatamab monotherapy in the real-word setting in relapsed-refractory multiple myeloma: results of the French compassionate use program on behalf of the IFM. Blood Cancer J. 2024;14:219.
Tan CR, Asoori S, Huang CY, Brunaldi L, Popat R, Kastritis E, et al. Real-world evaluation of teclistamab for the treatment of relapsed/refractory multiple myeloma (RRMM): an International Myeloma Working Group Study. Blood Cancer J. 2025;15:53.
Acknowledgements
This work was supported in part by the Cancer Center Support Grant (NCI Grant P30 CA016672). RZO, the Florence Maude Thomas Cancer Research Professor, would like to acknowledge support from the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, the Riney Family Multiple Myeloma Research Fund at MD Anderson from the Paula and Rodger Riney Foundation, and the MD Anderson Cancer Center High Risk Multiple Myeloma Moon Shot.
Author information
Authors and Affiliations
Contributions
OP and MHQ conceived and designed the study; OP collected and assembled the data; OP, CM, and DRM analyzed and verified the data; OP, CM, and MHQ wrote the manuscript. OP, CM, DRM, AAH, MRT, QB, SS, NS, PS, PL, JR, YN, AHM, URS, AJ, GT, YA, HCL, KKP, PK, SKT, RZO, REC, EJS, and MHQ interpreted the data, wrote and approved the article, and are accountable for the publication.
Corresponding author
Ethics declarations
Competing interests
HCL has received consulting fees from Bristol Myers Squibb, Alexion Pharmaceuticals, Janssen, Regeneron, GlaxoSmithKline, Sanofi, Takeda Pharmaceuticals, Allogene Therapeutics, Pfizer, and Menarini. HCL has also received research funding from Amgen, Bristol Myers Squibb, Janssen, GlaxoSmithKline, Regeneron, Takeda Pharmaceuticals, and Alexion Pharmaceuticals. All other authors report no conflicts of interest.
Ethics approval and consent to participate
This study was approved by the University of Texas MD Anderson Institutional Review Board (IRB) under protocol number PA17-0450. Approval was obtained from the IRB to waive informed consent for this retrospective chart review. The study was conducted in accordance with the Declaration of Helsinki and the 1996 Health Insurance Portability and Accountability Act.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pasvolsky, O., Marcoux, C., Milton, D.R. et al. Results of delayed or salvage autologous hematopoietic stem cell transplantation for multiple myeloma. Bone Marrow Transplant (2025). https://doi.org/10.1038/s41409-025-02771-3
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41409-025-02771-3